Materials for Thin Film SOFCs
Introduction
Fuel cells generate electricity through the oxidation of natural gas, hydrogen, or other fuels. Solid oxide fuel cells (SOFCs) utilize an oxide ion conducting solid oxide located between the electrodes as the electrolyte. The anode and cathode are typically comprised of porous ceramic composites with good electronic and ionic conductivity. The anode and cathode act as catalysts for fuel oxidation and oxygen reduction, respectively. Ideal electrolytes are dense and predominantly an ionic conductor, for example yttria stabilized zirconia (YSZ) (Prod. No. 572349).1 The use of a solid electrolyte in SOFCs offers distinct advantages owing to the lack of associated corrosion and electrolyte management problems associated with liquid electrolytes. SOFC technology has been developed for a wide variety of power generation applications such as auxiliary power units in vehicles to stationary power generation with outputs ranging from hundreds of watts to megawatts. The attractiveness of this technology is its efficient and clean production of electricity from a variety of fuels. The high operating temperatures of SOFCs also make them suitable candidates for application with heat engine energy recovery devices or combined heat and power systems, thereby further increasing the overall fuel efficiency.
Reducing SOFCs operating temperatures
The use of bulk ceramic electrolytes in standard SOFCs typically requires operation at temperatures >700 oC to achieve sufficient ionic conductivities. These high temperature requirements increase start-up time, place heavy stability demands on the materials, and limit their applications to large stationary sources. Therefore, research efforts are focused on lowering the operating temperatures of SOFCs below 600 oC. These lower temperature devices are called as intermediate-temperature SOFCs (IT-SOFCs). Achieving lower operating temperatures while maintaining high energy conversion efficiency will not only extend potential applications to transportation and portable power generation but also enable the use of lower cost metallic interconnects, reduce thermal stresses and decrease start-up times. However, reduced temperatures will also lead to increased electrolyte ohmic resistance losses arising due to the thermally activated behavior of ionic transport.2-4
Performance losses associated with lower operating temperatures can be compensated by decreasing the electrolyte thickness (Figure 1). Thin films of electrolytes help solve this problem with existing materials by
- reducing oxygen ions traveling distance and electrolyte resistance as resistance is inversely proportional to conductor length;
- producing grain structures that are less resistive such as columnar grain structure;
- controlling nano-crystalline micro-structure of thin films which enables fine-tuning of electrical conductivity; and
- depositing films with large interfacial areas as power output is also proportional to interfacial area.
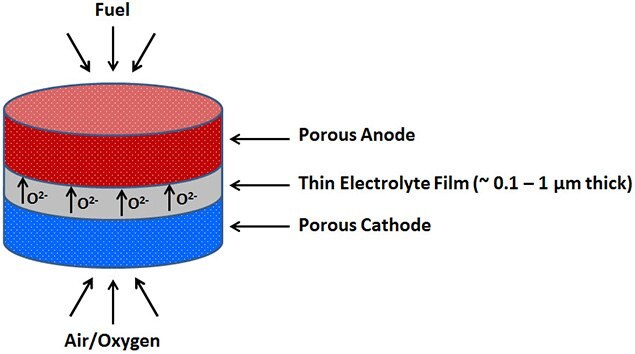
Figure 1.Schematic diagram of thin film solid oxide fuel cell (TF-SOFC)
Deposition of electrolyte thin films
SOFCs with thin films of electrolytes are often called as thin film solid oxide fuel cells (TF-SOFCs). Electrolyte thin films must be continuous and crack-free in order to prevent gas leakage, and must rest between two porous electrodes through which gases can pass freely to or away from the active sites for electrochemical reactions near the electrode-electrolyte interface.
Fabrication of TF-SOFCs requires the deposition of electrolyte thin films of 100 - 1000 nm thickness (Figure 1). Different technologies like chemical vapor or atomic layer deposition (CVD/ALD), electrochemical vapor deposition, various sputtering or physical vapor deposition (PVD) processes using ion beam, magnetron, electron beam and so forth, ink jet printing, and also sol-gel processing can be used to achieve thin film deposition. The choice of an appropriate thin film deposition technique is strongly influenced by the material to be deposited and the desired film quality.3-4
Among the various methods available for thin film deposition, CVD/ALD and PVD (sputtering) methods are the two most commonly employed techniques for deposition of electrolyte thin films. With CVD, dense electrolyte thin films can be formed at moderate temperatures while maintaining the uniformity and high purity of the films. For example, yttria stabilized zirconia (YSZ) is still one of the most commonly used electrolytes in SOFCs. YSZ thin films can be deposited by ALD method by alternating cycles of the standard ZrO2 and Y2O3 film deposition processes or by using a Zr/Y pulse of the desired ratio. More specifically, YSZ films containing 7-8 mol% yttria can be fabricated by sequentially depositing seven layers of zirconia from tetrakis(dimethylamido)zirconium precursor (Prod. No. 579211) and one layer of yttria from tris(cyclopentadienyl)yttrium precursor (Prod. No. 491969).4-6
Sputtering is another one of the widely used thin film deposition technique. By adjusting deposition parameters, for example, gas pressure, deposition power and rate, substrate temperature, and sputtering time, different morphologies including fully dense and highly porous films can be obtained. Y/Zr films of different porosities can be deposited by DC sputtering of Y-Zr alloy sputtering targets (Prod. No. 774057) and adjusting the deposition rates and Argon pressure. The Y/Zr films can then be oxidized in a box furnace by heating in air for extended periods of time to form the corresponding oxide (YSZ) films. Alternatively, YSZ films can also be obtained by RF magnetron sputtering of YSZ targets (Prod. No. 774049).7
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?