Determination of Tanshinone IIA, Notoginsenoside R1, Ginsenoside Rg1 and Ginsenoside Rb1 in Guanxin Danshen Diwan
Abstract
Two HPLC methods were developed for the determination of tanshinone IIA, notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1 in Guanxin Danshen Diwan, following a public draft monograph released by the Chinese Pharmacopoeia Commission. Following sample preparation, tanshinone IIA was analyzed using a Discovery® C18 column, whereas notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1 were determined with a Discovery® HS C18 HPLC column. The results demonstrated that both methods satisfied the requirements of the Chinese pharmacopeia draft, confirming their applicability for the quantification of the active ingredients in Guanxin Danshen Diwan.
Section Overview
Introduction
Guanxin Danshen Diwan (or: “Diwan”) is formulated from three herbal medicines: Dan Shen, San Qi, and Xiang Fu You.1,2 It is primarily used for the treatment and relief of chest pain associated with Qi stagnation and blood stasis, with symptoms including chest tightness, chest pain, palpitations, and shortness of breath.2,3
In 2024, the official website of the Chinese Pharmacopoeia Commission published a draft method for the detection of four active compounds in Guanxin Danshen Diwan: tanshinone IIA, notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1 (Figure 1).4 Upon successful public validation, this monograph will be incorporated into the 2025 edition of the Chinese Pharmacopoeia. In accordance with this draft, two methods were developed and evaluated for the quantitative determination of these four active compounds in a commercially available preparation of Guanxin Danshen Diwan, thereby supporting quality control in accordance with pharmacopeial requirements.

Figure 1.Structures of tanshinone IIA, notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1.
Experimental
Standard and Sample Preparation
The standards and sample were prepared according to the below-described procedures.
Standard Preparation
For the determination of tanshinone IIA:
- Stock solution: Weigh 10 mg of a tanshinone IIA reference standard into a 10 mL brown volumetric flask. Add ~8 mL methanol and sonicate for 5 minutes. Top up to the mark with the mobile phase and mix well. The resulting concentration for tanshinone IIA is 1 mg/mL.
- Standard solutions for external calibration: Dilute the stock solution with methanol to obtain a series of standard solutions with concentrations of 1, 2, 5, 10, 20, and 50 µg/mL for tanshinone IIA.
For the determination of notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1:
- Stock solution: Separately weigh 2.5 mg of a notoginsenoside R1, 10 mg of ginsenoside Rg1, and 10 mg of ginsenoside Rb1 reference standard into a 10 mL brown volumetric flask. Add ~8 mL methanol and sonicate for 5 minutes. Top up to the mark with the mobile phase and mix well. The concentrations in the resulting stock solution were 0.25 mg/mL, 1.0 mg/mL, and 1.0 mg/mL for notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1, respectively.
- Standard solutions for external calibration: Dilute the stock solution with 19% acetonitrile aqueous solution to obtain a series of standard solutions with concentrations of 2.5, 5, 12.5, 25, 50, and 125 µg/mL for notoginsenoside R1 and 10, 20, 50, 100, 200, and 500 µg/mL for ginsenoside Rg1 and ginsenoside Rb1.
Sample Preparation
Sample: Guanxin Danshen Diwan purchased from a local pharmacy.
For tanshinone IIA:
- Precisely weigh 50 tablets of Guanxin Danshen Diwan, and grind to a fine powder.
- Precisely weigh about 0.8 g of the powdered sample, and transfer to an amber glass bottle.
- Accurately add 25 mL of 80% methanol (80:20 methanol:water, v/v).
- Weigh the sample, sonicate at 250 W/40 kHz for 30 minutes, allow to cool, and weigh again.
- Use 80% methanol to make up for the weight loss and mix well.
- Filter through a 0.45 μm membrane filter before HPLC analysis.
For notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1:
- Precisely weigh 50 tablets of Guanxin Danshen Diwan, and grind to a fine powder.
- Precisely weigh about 0.4 g of the powdered sample, and transfer to an amber glass bottle.
- Accurately add 50 mL of 80% methanol (80:20 methanol:water, v/v).
- Weigh the sample, sonicate at 250 W/40 kHz for 30 minutes, allow to cool, and weigh again.
- Use 80% methanol to make up for the weight loss and mix well.
- Filter through a 0.45 μm membrane filter before HPLC analysis.
HPLC-UV Analysis
The Diwan sample extracts and corresponding standards were analyzed by HPLC-UV. Tanshinone IIA was determined using a Discovery® C18 (Table 1), whereas notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1 were analyzed on a Discovery® HS C18 HPLC column (Table 2).
Acceptance Criteria
Acceptance criteria for standard solutions
- Theoretical plate number calculated for the peak of tanshinone IIA: NLT (not less than) 3000.
- Theoretical plate number calculated for the peak of ginsenoside Rg1: NLT 4000.
- The resolution between tanshinone IIA, notoginsenoside R1, ginsenoside Rg1 and, ginsenoside Rb1 and their closest sample impurity peaks must not be less than 1.5 (Ch. P. HPLC method general rule 0512).
Results & Discussion
Chromatograms obtained for the developed HPLC methods for the standard solutions are displayed in Figures 2 & 3, and the corresponding chromatographic data for tanshinone IIA, notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1 is summarized in Tables 3 and 4. The theoretical plate count was 11,114 for tanshinone IIA and 73,624 for ginsenoside Rg1. Both values exceeded the requirements of the Chinese pharmacopeia draft (NLT 3000 and 4000, respectively), confirming that the two developed methods can be utilized for the quantification of the four active ingredients in Guanxin Danshen Diwan in accordance with the draft monograph.
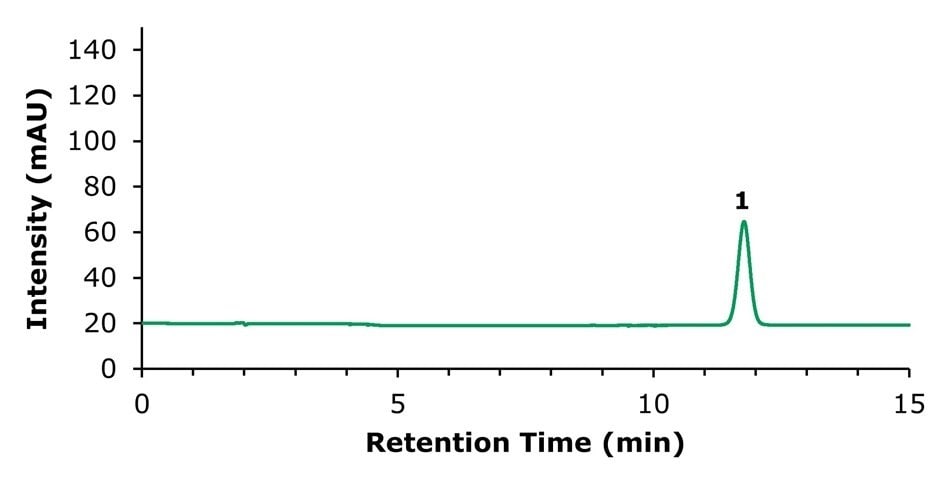
Figure 2.HPLC-UV chromatogram of the standard solution containing 20 µg/mL of tanshinone IIA (1).
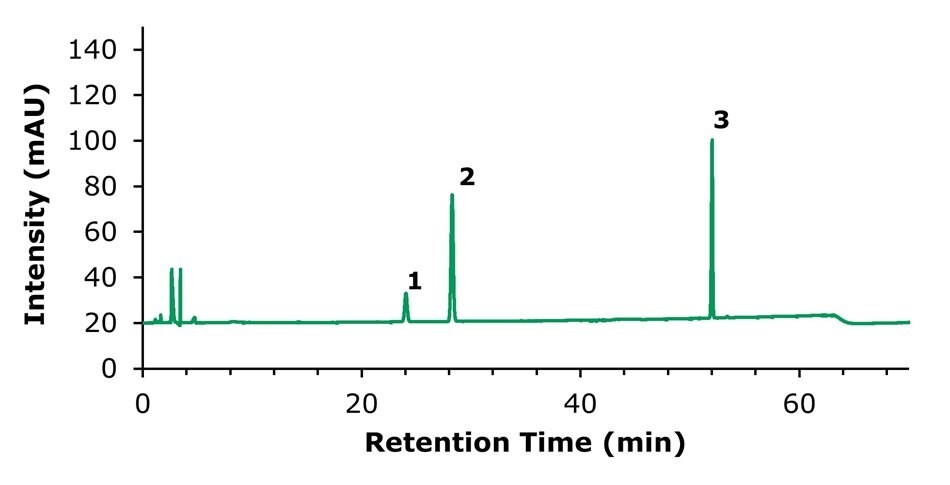
Figure 3.HPLC-UV chromatogram of the standard solution containing 50 µg/mL of notoginsenoside R1 (1), and 200 µg/mL of ginsenoside Rg1 (2) and ginsenoside Rb1 (3), respectively.
Calibration and Repeatability
Calibration was performed in accordance with the draft monograph method. The curve for tanshinone IIA is shown as a representative example in Figure 4. Similar results were obtained for the other compounds, with the exception of notoginsenoside R1, where the 2.5 μg/mL standard produced a signal too low for quantification and was therefore excluded from the calibration range. A summary of the calibration data is provided in Table 5. All compounds demonstrated good linearity, with R² values greater than 0.99. Repeatability testing for the two methods yielded relative standard deviation (RSD) values ranging from 0.34% to 1.63% (Tables 6 and 7).
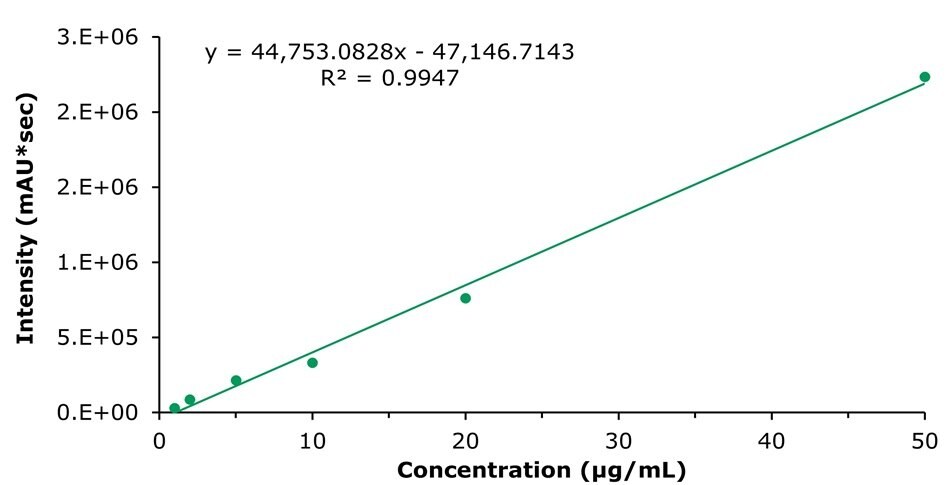
Figure 4.Calibration curve obtained for tanshinone IIA.
Sensitivity
The sensitivity of the developed methods is summarized in Table 8. The LOD and LOQ were determined based on the lowest concentrations of the calibration standards.
Tablet Sample Analysis
Chromatographic separations of the tablet sample preparations are shown in Figures 5 & 6, and the corresponding chromatographic data is summarized in Tables 9 & 10. All neighboring impurity peaks originating from the tablet matrix were adequately resolved from the analytes of interest, with a resolution >1.5, thereby meeting the requirements of the Ch.P. HPLC method general rule 0512.
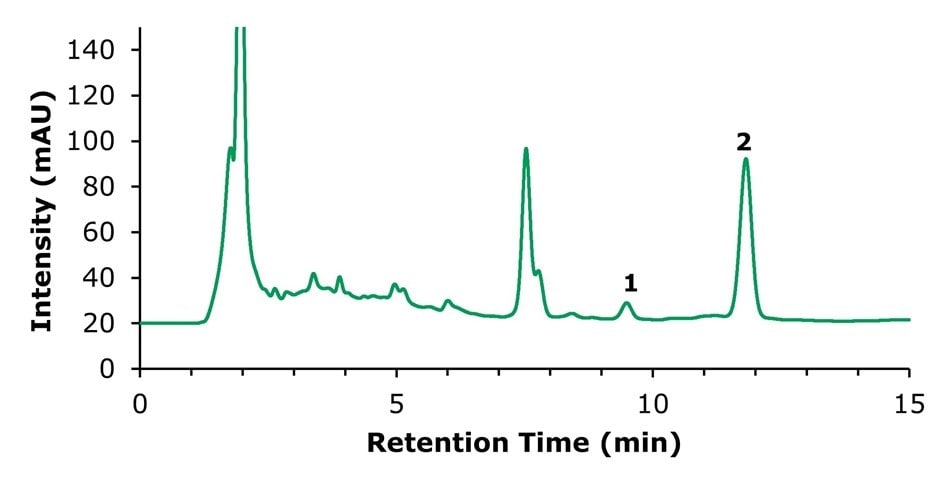
Figure 5.HPLC-UV chromatogram of the sample solution used for the quantification of tanshinone IIA (2).
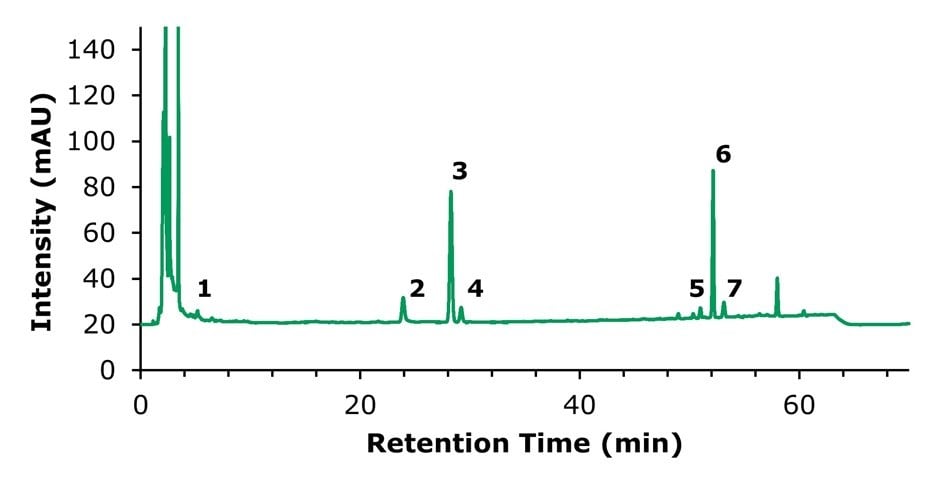
Figure 6.HPLC-UV chromatogram of the sample solution used for the quantification of notoginsenoside R1 (2), ginsenoside Rg1 (3), and ginsenoside Rb1 (6).
Conclusion
Two HPLC-UV methods were developed in accordance with the draft Chinese pharmacopeia monograph for the analysis of the four active compounds tanshinone IIA, notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1 in Guanxin Danshen Diwan. The powdered samples were extracted with 80% aqueous methanolic solution, and the resulting extracts were filtered and analyzed using Discovery® C18 and Discovery® HS C18 columns.
The developed method exceeded the theoretical plate counts required by the Chinese Pharmacopoeia acceptance criteria and fulfilled the resolution criteria outlined in the Ch. P. HPLC method general rule 0512. These results confirm the applicability of the two columns for the efficient, sensitive, and reproducible analysis of the active ingredients in Guanxin Danshen Diwan, supporting their use for pharmacopeial quality control.
Solvents, Reagents & Accessories
Reference Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?