优化生物处理的客户合作实验室
解决您最棘手的工艺难题。
当您急于将提高生命质量的疗法推向市场时,即使是很小的问题也会造成很大的延误--除非您有积极主动的解决方案。
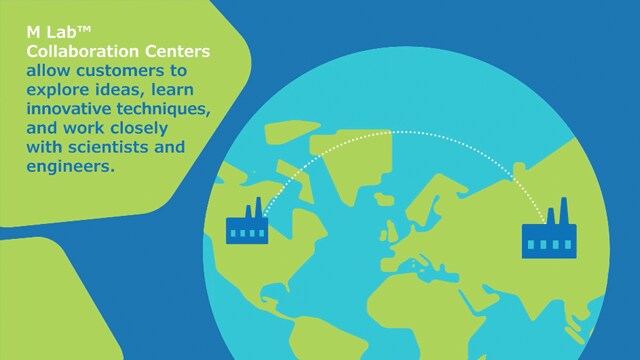
这就是您在 M Lab™ 卓越生物制造协作中心所能找到的:与顶尖科学专家进行面对面或虚拟协作,探索创新的生物加工技术,以及充满活力的实验室空间,以真实再现您的工艺培训。
这不仅仅是一种资源,更是一种体验,帮助您更快地将产品提供给更多患者。
在 M Lab™ 协作中心了解、优化和扩展您的生物工艺
每一个 M Lab™ 协作中心的体验都是为满足您的独特需求而量身定制的。
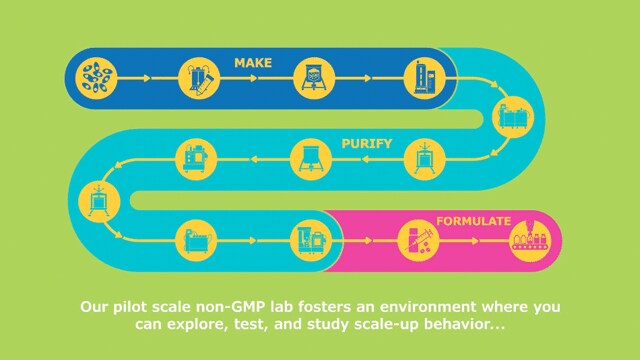
- 在多模式、非 GMP 环境中探索 新技术。开发和优化整个生物制药工艺流程,而无需中断自身运营。
- 了解生物制药工艺 4.0 如何加快您的产品上市速度。
- 与 300 多位科学领军人物合作。
在整个工艺流程中利用动态技术经验提升您的生物工艺团队。
生物/制药生产技术和法规在不断发展。
这正是您将从北美和全球提供的生物制造培训机会中获得的。平均拥有 20 年经验的技术培训讲师将通过各种授课平台帮助您的团队成长,并加强他们的上游和下游技能:
- 面对面 → 无论是在您的工厂还是在 M Lab™ 协作中心的某个地点,都可以进行生物制造实践培训
- Livestream → For an interactive learning experience delivered from our lab to yours
- E-Learning → For digital, on-demand access from anywhere
测试您的想法(而不会让您的生物加工操作面临风险)
无论您是在开发新的分子,还是在重新构想既定的生产策略,M Lab™ 协作中心都能为您提供一个灵活的空间,让您对新的生物制药生产工艺充满好奇,掌握最佳实践,并在做出实际决策之前优化您的放大方法。
为您的生物技术初创企业的未来保驾护航
通过专家提供的个性化支持,避免在商业化道路上出现意外。M Lab™ 协作中心利用我们的生物技术资源中心为您提供完整的解决方案。
随时随地为任何问题设计生物工艺解决方案
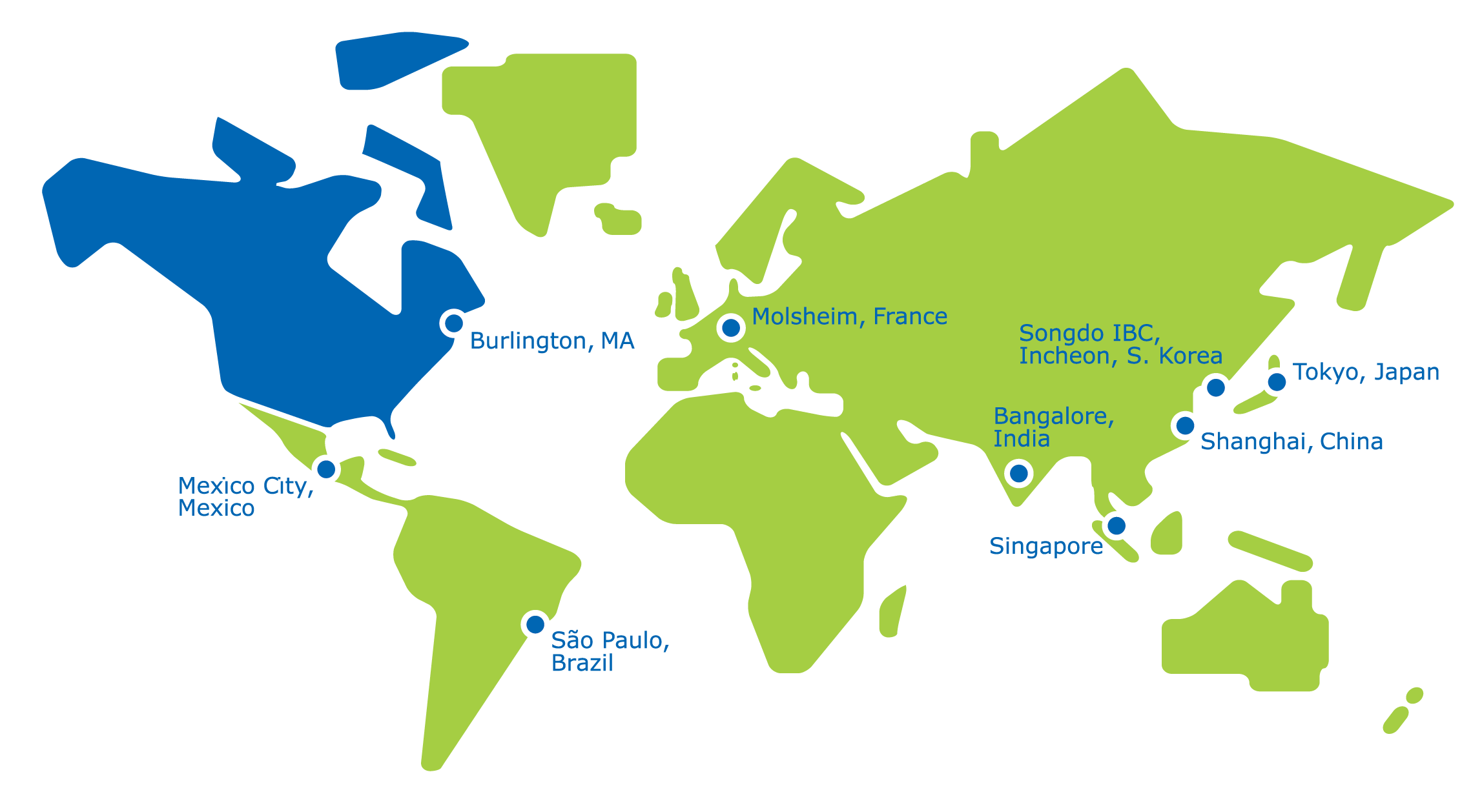
参观位于您附近的全球生物技术中心的 M Lab™ 协作中心,或通过无缝的世界级远程技术进行虚拟协作。
相关资源
- Brochure: M Lab™ Collaboration Center
A global network of vibrant collaboration labs where Life Science innovators explore big ideas, learn new techniques, and rapidly move from complex problems to optimal solutions–together.
- Podcast: Collaborations in Bioprocessing
In the podcasts below, you can listen to compelling stories of novel solutions and innovative application of technologies that brought M Lab™ Collaboration Centers team members together with various biopharmacuetical manufacturers to make science, better.
- Educational Brochure: Biopharmaceutical Course Brochure
Enhance your skill set by taking practical, hands-on bioprocessing and formulation courses at our M Lab™ Collaboration Centers, or invite our trainers to your site.
- Flyer: Collaborate for Success in Pharmaceutical Manufacturing
With the M Lab™ Collaboration Centers’ flexible, non-GMP environment and global network of technical expertise, you can solve your toughest problems, from adoption of single-use technologies to virus detection and removal, and expansion in emerging markets.
- Article: Tapping into Collective Intelligence: Why Collaboration is Critical for the Adoption and Implementation of Next-generation Bioprocessing
Next-generation bioprocessing is a step-by-step evolution already well underway as the pharma industry undergoes a paradigm shift toward continuous processing.
- Report: Biopharma's Evolution: Learnings from the Pandemic for a Revived Regulatory Landscape
It took seven months from clinical trials commencing for the first covid-19 vaccine to be made available for public use. Pre-pandemic, the median time to market for a biotherapeutic treatment was nine years and four months, and the fastest had been four years for mumps in the 1960s.
如要继续阅读,请登录或创建帐户。
暂无帐户?