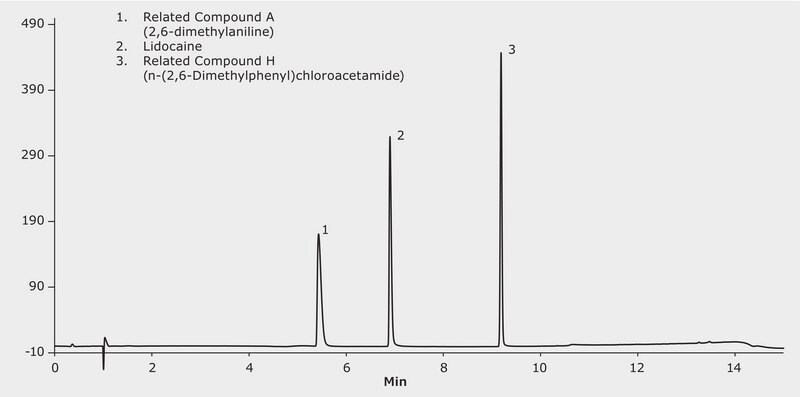
HPLC Analysis of Lidocaine and Related Compound A and H on Purospher®STAR RP-18 endcapped
材料
used together
CONDITIONS
column
Purostar RP18E 2MYM HI-HR100-2.1 mm (1.50648.0001)
mobile phase
[A] 95/5 water / acetonitrile in 0.1% phosphoric acid; [B] 5/95 water/acetonitrile in 0.1% phosphoric acid
gradient
0% B for 2 min, then 0% to 100% B in 10 min; 100% to 0% B in 0.1 min; hold at 0% B for 4.9 min.
flow rate
0.3 mL/min
pressure
4656 psi (321 bar)
column temp.
30 °C
detector
210 nm UV
injection
1 μL
sample
100 μg/mL of lidocaine, related compound A and H in acetonitrile
说明
分析说明
Lidocaine is a medication used to numb tissue in a specific area and does not affect other areas. Topically it can be used for reducing pain by irritations such as sunburn, poison ivy, insect bites, minor cuts or even hemorrhoids. It can also be used for local anesthetic with sedative, analgesic and cardiac depressant (antiarrhythmic drug) properties This application demonstrates reverse phase interactions in a simple gradient separating lidocaine and two of its related compounds using Cerilliant CRM′s for the related compounds.
法律信息
Purospher is a registered trademark of Merck KGaA, Darmstadt, Germany