Fluorescent Lipid Probes - Avanti Research™
Illuminate Your Research with Fluorophores

Figure 1.Discover Avanti Research™ fluorophores.
Fluorescent probes are essential tools for visualizing biological systems, enabling researchers to track molecules, monitor disease markers, and study cellular function with high sensitivity and minimal disruption. By absorbing and emitting light at specific wavelengths, these fluorophores reveal valuable insights, even at low concentrations.
As the exclusive partner for Avanti Research™ products outside the U.S. and Canada, we offer one of the most comprehensive selections of fluorescent and lipid-conjugated probes, spanning the full emission spectrum. The portfolio includes widely used fluorophores such as FITC, Rhodamine, TopFluor™ (BODIPY), NBD, Cy3, Cy5, and more, giving you the flexibility to target a broad range of biomolecules across diverse applications.
Explore our fluorescent probe solutions and bring your research into focus. With optimized fluorescence, achieve bright signals and clear, confident results.
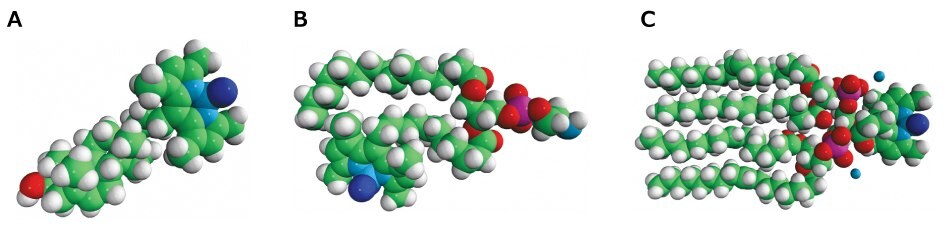
Figure 2.Three examples of offerings in our ever growing fluorescent lipids portfolio: a) TopFluor™ Cholesterol; b) TopFluor™ PE; and c) TopFluor™ Cardiolipin
Fluorescent Probes:
NBD & TopFluor™
Nitrobenzoxadiazole (NBD)
Environmentally sensitive and easily modified, NBD reacts with amines and thiols, making it ideal for labeling lipids, studying membrane dynamics, and probing biological nucleophiles in live systems.
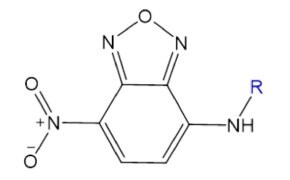
Figure 3.Structure of fluorophore nitrobenzoxadiazole (NBD).
TopFluor™ (BODIPY)
TopFluor™ (BODIPY) is a highly versatile, photostable fluorophore with tunable emission (500–700 nm) and strong quantum yields across environments. Its minimal impact on lipid behavior makes TopFluor™-labeled lipids ideal for studying membrane dynamics, lipid diffusion, and cellular localization.
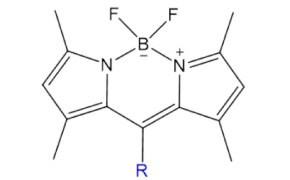
Figure 4.Structure of Top Fluor™.
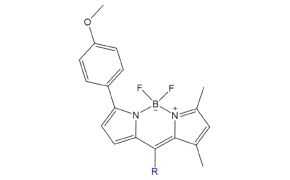
Figure 5.Structure of Top Fluor TMR™.
Fluorescent Probes: Cyanines & Squaraines
Cyanines
Cyanine probes are fluorescent dyes composed of two indole rings linked by a variable-length polyalkene chain, with longer chains shifting excitation and emission to longer wavelengths. Known for their high photostability and water solubility, cyanine dyes are ideal for labeling amines on proteins, antibodies, and nucleic acids for detection via microscopy or flow cytometry.
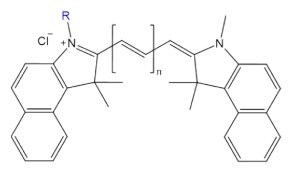
Figure 6.Structure of fluorescent probe cyanine n.5, where n = carbon linker length.
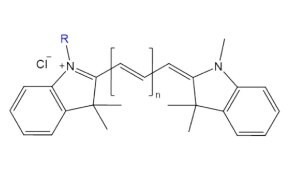
Figure 7.Structure of fluorescent probe cyanine n, where n = carbon linker length.
SquareFluor™ (Squaraines)
SquareFluor™ (squaraine) probes feature a squaric acid-derived aromatic ring that enables intense absorption, strong photostability, and high quantum yield. Once limited by sensitivity to nucleophilic attack, recent design improvements have renewed interest in their use for bioimaging, photodynamic therapy, and analyte sensing.
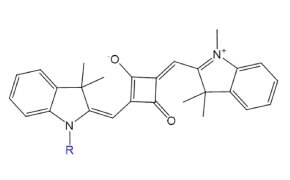
Figure 8.Structure of fluorescent probe SquareFluor™ (Ex/Em: 610/630 nm).
Liss Rhod, DPH, & Pyrene
Lissamine Rhodamine (Liss Rhod)
Lissamine Rhodamine (Liss Rhod) is a fluorescent probe commonly used to study cell membrane dynamics, membrane fusion, and liposome behavior. Its strong fluorescence makes it ideal for visualizing lipid interactions, with tagged lipids like PEs, fatty acids, and triglycerides available for a range of applications.

Figure 9.Structure of fluorescent probe Lissamine Rhodamine (Liss Rhod).
DPH
1,6-Diphenyl-1,3,5-hexatriene (DPH) is a hydrophobic fluorescent probe widely used to study membrane dynamics and lipid bilayer structure via fluorescence anisotropy. Its weak fluorescence in water but strong emission in hydrophobic environments makes it ideal for assessing lipid tail order, membrane fluidity, and interfacial properties.
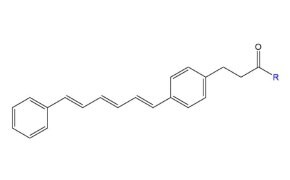
Figure 10.Structure of fluorescent probe DPH (1,6-Diphenyl-1,3,5-hexatriene).
Pyrene
Pyrenedecanoyl (pyrene) is a widely used fluorescent label known for its ability to form excimers, excited-state dimers with red-shifted emission around 470 nm. This unique property makes pyrene-labeled lipids ideal for studying membrane fusion, phospholipid transfer, protein folding, and structural changes in biomolecules.
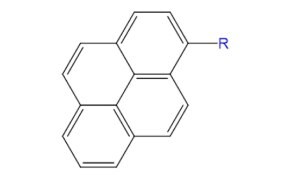
Figure 11.Structure of fluorophore pyrenedecanoyl (pyrene).
Dansyl, Laurdan, and Fluorescein
Dansyl
Dansyl probes are fluorescent labels often used to modify proteins and amino acids, offering high quantum yield and large Stokes shifts for easy detection. They are also useful in heavy metal sensing due to electron transfer interactions with metal ions.
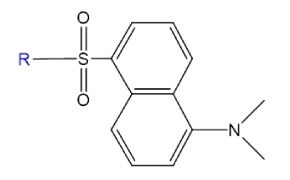
Figure 12.Structure of fluorophore danysl.
Laurdan
Laurdan is a polarity-sensitive fluorescent probe whose emission spectrum shifts based on the polarity of its surrounding environment. This unique responsiveness makes it a powerful tool for investigating membrane order and lipid bilayer dynamics.
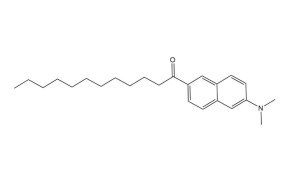
Figure 13.Structure of fluorescent probe laurdan.
Fluorescein
Fluorescein is a widely used fluorescent probe known for its strong green emission and versatility in biological applications. Beyond its use in ophthalmology, it aids in bioimaging and tissue differentiation, with derivatives like FITC commonly used for protein labeling and antibodies.
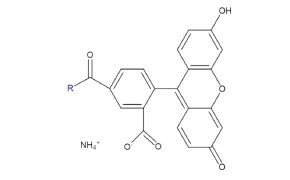
Figure 14.Structure of fluorescent probe fluorescein.
Functionalized Probes
Azide functionalized fluorescent probes
Azide-functionalized fluorescent probes enable copper-catalyzed click chemistry reactions with other molecules containing “acceptor” groups, such as alkynes.

Figure 15.Structure of an azide-functionalized molecule.
Maleimide functionalized fluorescent probes
Maleimide is especially effective for conjugating fluorescent probes to proteins or peptides with cysteine residues, offering a selective and efficient method to attach the probe to molecules containing thiol functional groups.

Figure 16.Structure of fluorophore maleimide.
DBCO functionalized fluorescent probes
Fluorescent probes functionalized with DBCO enable copper-free click chemistry by reacting with molecules that have “donor” groups like azides.
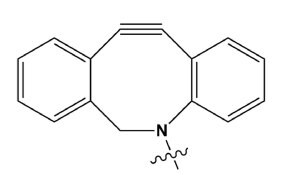
Figure 17.Structure of DBCO.
NHS functionalized fluorescent probes
Featuring an activated NHS ester moiety, these fluorophores readily react with nucleophiles like amines. NHS esters can bind to amines under mild alkaline conditions without needing additional activation. These probes are commonly used for fluorescent labeling of peptides or proteins.
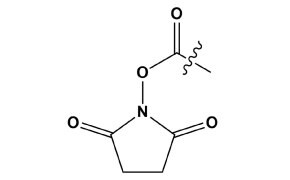
Figure 18.Structure of an NHS ester moiety.
Free acid functionalized fluorescent probes
Fluorescent probes functionalized with free acid groups contain a carboxylic acid moiety that reacts with primary amines to form stable amide bonds.

Figure 19.Structure of a free acid group.
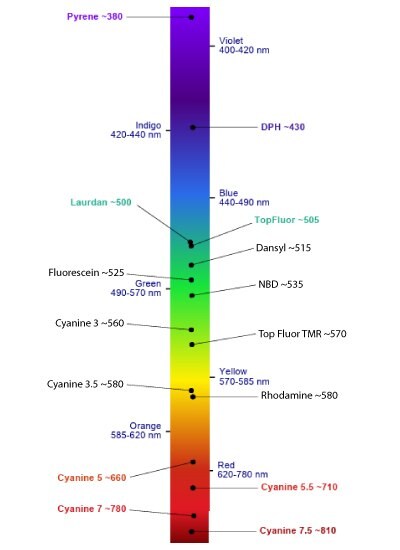
Figure 20.Emission Spectrum Guide provided by Avanti Research™.
Discover the complete fluorescent lipid portfolio here.
Explore our full range of biochemical solutions.
*All chemical structures provided by Avanti Research™.
Related Products
如要继续阅读,请登录或创建帐户。
暂无帐户?