How Ultrasensitive Biomarker Detection Platforms Accelerate Drug Development Research
Drug development research typically involves a huge investment of both time and resources. Learn how ultrasensitive biomarker detection platforms, such as the SMC® technology platform, can enable the acceleration of drug development research programs.
Drug Development Research Pipeline
Drug development research programs can span many years because drug candidates have to go through multiple stages before reaching the market, such as discovery, preclinical, and clinical research phases. They also involve the evaluation of drugs through pharmacokinetics (PK), pharmacodynamics (PD), and immunogenicity testing. This can involve quantifying markers to help understand drug behavior, find the optimal dosage, and understand safety and efficacy parameters. A typical timeline of this pipeline is shown in Figure 1.
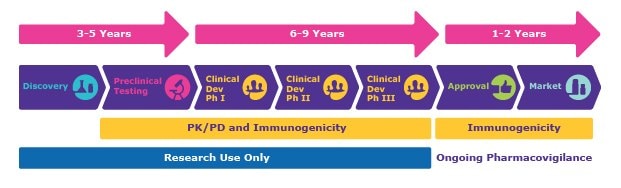
Figure 1.A timeline of a typical drug development research program. Pharmacokinetic/pharmacodynamic (PK/PD) and immunogenicity testing are critical from preclinical studies through Phase III, with continued immunogenicity assessment and pharmacovigilance activities occurring post-approval.
Key Drug Development Stages
- Discovery: Target identification, receptor binding, lead optimization
- Preclinical Testing: Proof of mechanism, ADME studies, dose ranging, PK/PD modeling, safety/toxicology profiling
- Clinical Development: Further safety/efficacy testing, dose selection, bioavailability testing
- Approval/Market: Ongoing safety monitoring, post-market surveillance, ongoing pharmacovigilance
Testing for PK/PD and immunogenicity are critical in most stages for the thorough evaluation of drug candidates to researchers make more informed go/no-go decisions.
PK/PD Testing
PK/PD testing helps determine how the drug is working in the body and how it should interact in the environment. The differences between PK and PD are described in Table 1 and Figure 2. Both of these parameters help inform critical decisions around dosing, safety, and efficacy measures.
Figure 2 below depicts how PK and PD interact across the drug development continuum from dose and exposure to efficacy, safety, and treatment adherence.
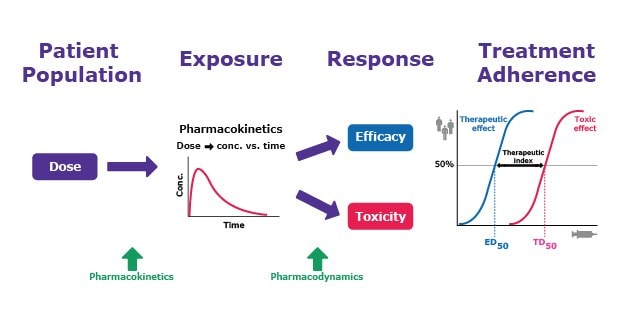
Figure 2.Understanding drug and effector response through pharmacokinetic and pharmacodynamic relationships.
Integrating Ultrasensitive Detection for Complete PK/PD Profiling
The goal of PK/PD testing is to deliver the right dose and achieve optimal drug exposure/effect. SMC® technology allows for the quantification of both low- and high-abundant biomarkers providing a wide dynamic range supporting:
- More accurate exposure–response modeling
- Reliable correlation of PK and PD parameters
- Early identification of efficacy and toxicity thresholds
Learn more about how ultrasensitive detection platforms can provide complete PK/PD profiling.
Immunogenicity Testing
Immunogenicity testing measures the immune response to a biotherapeutic. It involves the detection of anti-drug antibodies (ADA) and neutralizing antibodies (NAb). This helps determine if a drug will fail to get approved by giving an indication of efficacy, treatment outcomes, and any adverse effects such as hypersensitivity, infusion reactions, or anaphylaxis. By performing immunogenicity testing early, researchers decrease the failure rate of drugs and save time and money. Discover more about immunogenicity and how ultrasensitive detection platforms are critical for early assessment.
Ultrasensitive Detection Platform for Drug Development Research
Ultrasensitive immunoassay detection enables researchers to quantify low-abundant biomarkers with exceptional precision and reproducibility. The SMC® platform was designed to meet the demands of drug development research.
Key Features:
- Femtogram-level sensitivity
- Broad dynamic range (4–5 logs)
- Rapid sample reading time
- No-fluidics, low-maintenance
- Automation-compatible workflow
- Integrated Belysa® data analysis software
- Single data output for simple export to LIMS
- IQ/OQ/PQ validation services
For Research Use Only. Not For Use In Diagnostic Procedures.
如要继续阅读,请登录或创建帐户。
暂无帐户?