In Vitro Expansion of CD34+/CD38- Human Hematopoietic Progenitor Cells in a Serum-free and Xeno-free Stem Cell Culture Media
Hematopoietic stem cells (HSCs) represent a heterogeneous population of primitive blood progenitor cells that fall into several different classes.1,2 Within the human body, they are mainly located in the bone marrow of adults, but are also found in various fetal tissues such as umbilical cord blood, placenta and fetal liver. HSCs are functionally defined by their self-renewal capacity and their multipotency allowing the replenishment of all types of blood cells. The myeloid branch of their descendants is represented by monocytes/macrophages, granulocytes (neutrophils, basophils, eosinophils), erythrocytes, megakaryocytes (platelet producer cells) and dendritic cells. The lymphoid branch comprises of T-lymphocytes, B-lymphocytes and NK-cells.3 Treatments using HSCs (bone marrow transplantation) have been used for over 40 years now and are well-established.4 Indeed, HSCs still hold great potential for further applications in regenerative medicine and are therefore intensively investigated by the scientific community.
It is known that in vivo HSCs are able to expand to very large numbers by virtue of their pronounced self-renewal abilities.5 The in vitro colony forming unit (CFU) assay has been used to study the proliferation and differentiation pattern of hematopoietic progenitors by their ability to form colonies in a semisolid agar medium. However, researchers face difficulties during in vitro expansion of undifferentiated HSCs, with increasing differentiation of the cells observed during the expansion phase in traditional serum-containing culture systems. The establishment of serum-free but undefined formulations provided a somewhat more consistent culture environment, but the results were still not acceptable.
The PromoCell HPC Expansion Medium DXF (C-28021) provides a highly optimized, chemically defined and serum-free culture system for CD34+/CD133+ human hematopoietic stem cells. Optimal support of self-renewal and inhibition of differentiation results in superior expansion of human hematopoietic progenitor cells.
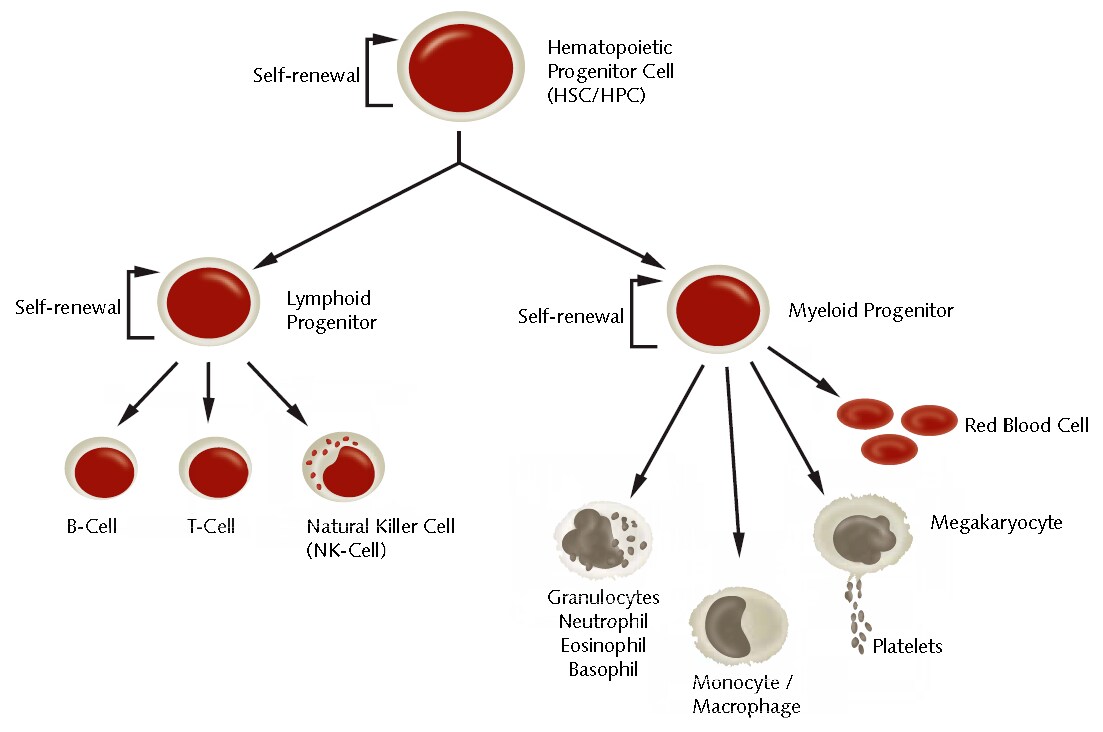
Figure 1.Differentiation of hematopoietic stem cells into various blood and immune cell types.
Hematopoietic Stem Cells Culture Protocol
- Prepare the Expansion Medium. Combine the Basal Medium and the Supplement Mix of the PromoCell HPC Expansion Medium DXF (C-28021) according to the instructions. Then, add an appropriate amount of PromoCell Cytokine Mix E to obtain the completely supplemented Expansion Medium.
- Seed HPCs. Pre-equilibrate an appropriate amount of the supplemented medium in the incubator at 37 °C and 5% CO2 for 30 minutes.
Freshly isolated HPCs: Plate the HPCs in the pre-equilibrated medium at a density of 5X103 cells/ml.
Cryopreserved HPCs: Thaw the cells for 2 minutes in a waterbath (refer to the PromoCell Instruction Manual for Human CD34+ Progenitor Cells for details). After thawing, immediately transfer them into the pre-equilibrated medium at a density of 5,000 cells per ml. Use at least 9 ml of medium per vial of cryopreserved cells. Leave the cells untouched in an incubator at 37 °C and 5% CO2 for 4-8 hours. Then, perform a complete medium change as described in the subcultivation section of the PromoCell Instruction Manual for Human CD34+ Progenitor Cells. Briefly, spin the cells down at 240 x g for 10 minutes, aspirate the supernatant and resuspend the cell pellet in fresh medium. - Expand the undifferentiated HPCs. Incubate the cells for 4 days at 37 °C and 5% CO2. For a partial medium change, remove the cells from the incubator. To create a single cell suspension pipet up and down several times and transfer the whole content of the tissue culture vessel into a 50 ml conical tube. Spin the cells down for 10 min at 240 x g. Then, carefully aspirate the upper two thirds of the medium. Gently resuspend the cells in the remaining third of the medium and replenish to the original volume with fresh cytokine-supplemented HPC Expansion Medium DXF (C-28021). Incubate for another 6-8 days to allow sufficient expansion of the cells. Replace two thirds of the medium as described above every 3 days.
- Harvest expanded HPCs. Harvest cells by collecting the medium from the tissue culture vessel containing the expanded HPCs. Pipet up and down several times in order to release loosely attached cells and to obtain a single cell suspension. Spin down the harvested HPCs at 240 x g for 10 minutes and discard the supernatant.
- Resuspend and count the cells. Resuspend the cells in PromoCell HPCs Expansion Medium DXF (C-28021) and count them. The HPCs are now ready to be used in your experiments, e.g. the differentiation into lineages of mature blood cells. The non-directed differentiation of Hematopoietic Cells into mature blood cells can be achieved using the PromoCell Hematopoietic Progenitor Medium (C-28020). In order to direct the differentiation process into specific lineages of mature blood cells, the PromoCell Hematopoietic Progenitor Medium may be supplemented with appropriate cytokines by the user.
- Characterize the cells. Characterize the hematopoietic immuno-phenotype of the expanded hematopoietic stem cells, e.g. by flow cytometry analysis. Primitive HPCs exhibit a CD34+/CD38- phenotype (Figure 2) and stain moderately positive for CD45.
Results
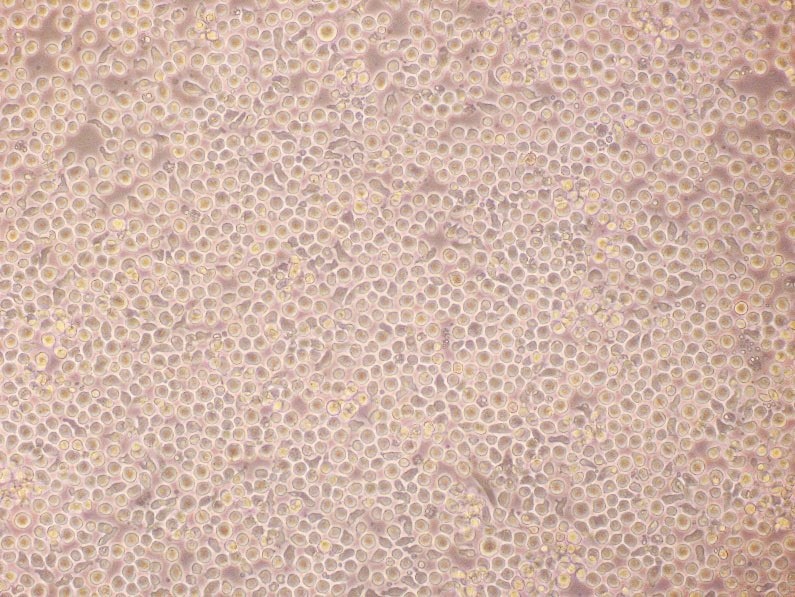
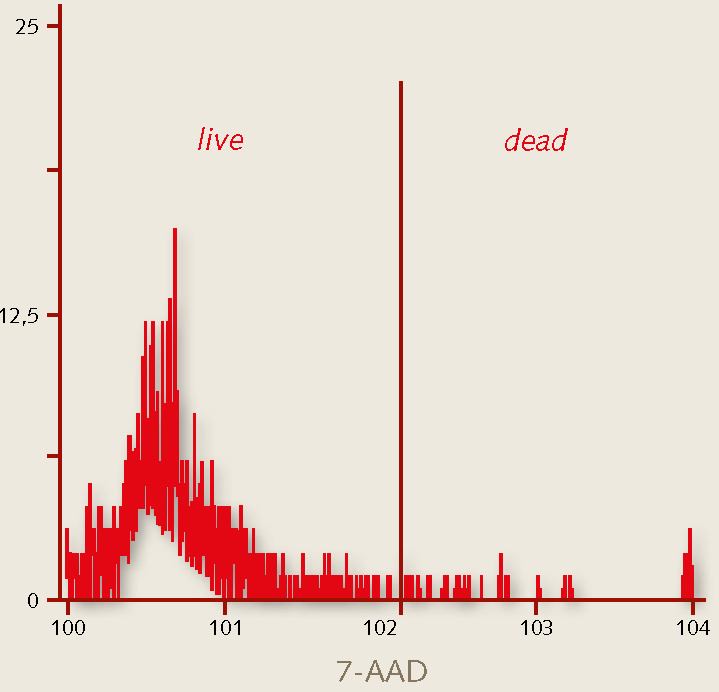
Figure 2. Expansion of human hematopoietic stem cells. Phase bright morphology and flow cytometry analysis of cord blood derived CD34+ progenitor cells after 12 days of expansion in PromoCell HPC Expansion Medium DXF. More than 98% of the cells are viable after the expansion period as detected by 7-AAD staining.
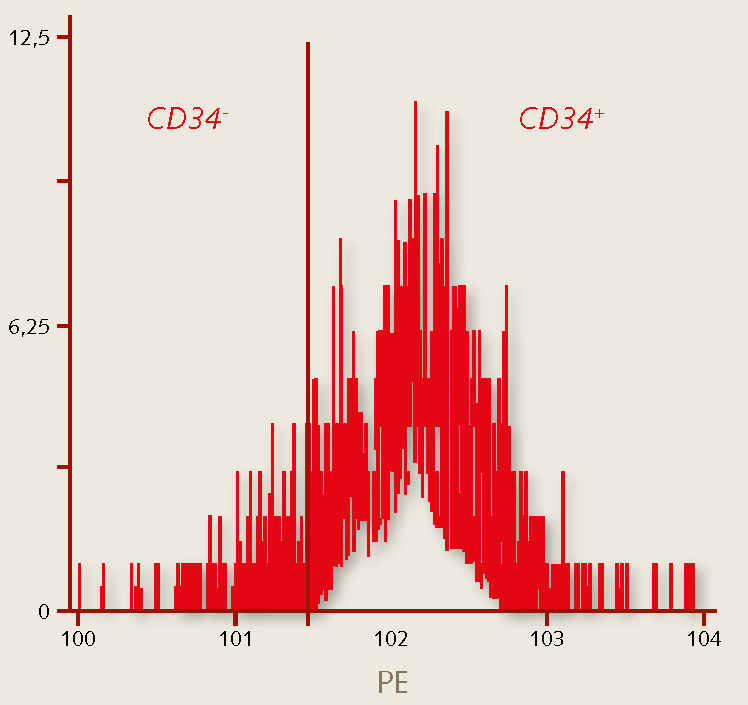
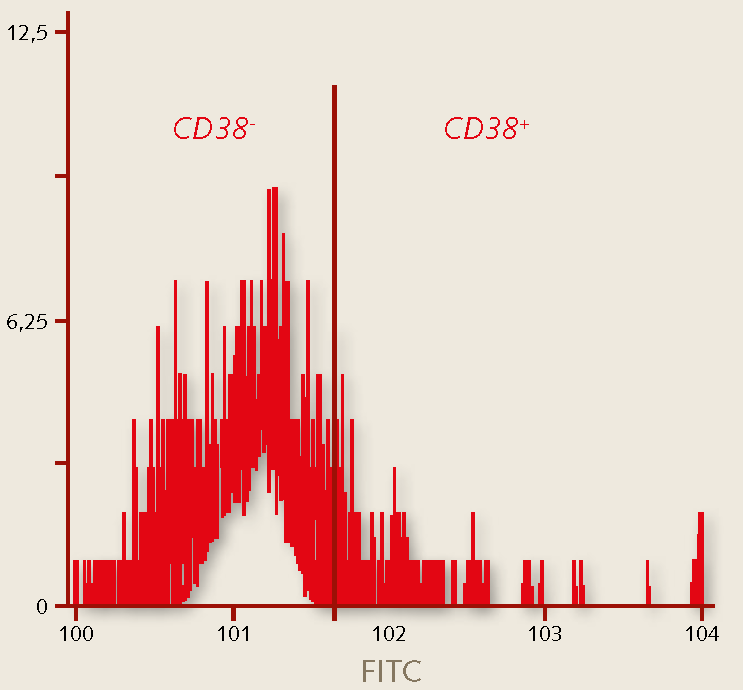
Figure 3. Characterization of human hematopoietic stem cells. The percentage of hematopoietic stem cells grown in the PromoCell HPC Expansion Medium DXF stain positive for CD34 (>93%), while >95% of the cells do not stain for CD38. The CD34+/CD38- marker profile indicates the maintenance of a primitive hematopoietic phenotype.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?