推荐单核细胞标准分离方法
基于Ficoll-Paque产品的细胞分离可在宽泛的血液样品体积中进行。由于其高产率,该方法可适用于处理极少量的血液,例如从儿童获得的样品。为了获得高分离可重复性,建议使用标准化操作步骤。以下操作步骤通过了我们的实验室使用Ficoll-Paque PLUS的评估并建议用于正常血液样本的分离,经过简单的更改即可适应特定的离心系统。该步骤也非常适合采用Ficoll-Paque PREMIUM、Ficoll-Paque PREMIUM 1.084以及Ficoll-Paque PREMIUM 1.073进行细胞分离。
为了建立标准化步骤,应先选择离心管的血容量和直径。这些因素决定了血液样本在管中的高度,进而决定了离心时间。增加管中血样的高度会增加红细胞的污染。然而分离却不会因为管直径的改变而受到明显影响。因此,在管中血样的高度和分离时间保持不变的情况下,管径越大,相同的纯化程度下分离体积就越大。
单核细胞的产量和纯化程度很大程度取决于红细胞的去除效率。
当全血中的红细胞发生聚集时,一些单核细胞会被捕获在团块中而与红细胞一同沉淀。这种单核细胞捕获的倾向性可通过稀释血液来减少。稀释有利于提高单核细胞产量并降低红细胞团块的大小。红细胞的聚集在较高温度(37℃)下会增强,从而会降低单核细胞的产量。然而在较低温度(4°C)下,聚集速率减低但会增加分离时间,这也会使得单核细胞的产量发生降低。折衷温度在18ºC至20°C中间可获得最佳效果。
自备设备和溶液
- 无菌平衡盐溶液或其他标准盐溶液(参考“试剂制备”部分)。
- 带有水平转子的离心机(应关闭制动)。
- 无菌离心管和移液器。
- 无菌针头和注射器。
- 相应选择的红细胞裂解液(如果分离粒细胞)。
试剂制备
稀释及洗涤液
用于血液稀释和细胞洗涤的平衡盐溶液可按照以下指南进行制备。其他的稀释液和洗涤液如等压无Ca2+/Mg2+的磷酸盐缓冲液(如杜氏PBS)、盐溶液(如Hank)或细胞培养培养基(如RPMI 1640)也可使用。
平衡盐溶液
在进行平衡盐溶液制备时,将一份体积的储存液A与九份体积的储存液B混合后进行灭菌。至少20 ml的每个样本需要被处理。可能也会用到其他无菌的标准盐溶液。
溶于约950 ml的蒸馏水中并加入浓盐酸至pH为7.6,然后将体积调整为1L。
在进行平衡盐溶液制备时,将一份体积的溶液A与九份体积的溶液B进行混合。溶液应每周新鲜制备。其他标准盐溶液可能也会被用到。
Ficoll-Paque产品
在使用前将Ficoll-Paque密度梯度培养基预热至18°C到20°C。对于大于3 ml的样品,请参照备注。
样品制备
应当使用新鲜的血液以确保分离的单核细胞的高活性。样品应在18°C到20°C间进行制备。
- 或抗凝处理的血液以及等体积的平衡盐溶液(终体积为4ml)。
- 倒置管数次将血液和缓冲液混匀,或通过上下吹打将混合液混匀。
单核细胞分离操作步骤
- 将Ficoll-Paque培养基瓶倒置数次充分混匀。
如果通过注射器吸取Ficoll-Paque培养基:将聚丙烯盖子拉开并将注射器针头穿过隔垫(图1)。
Ficoll-Paque培养基:取下折断式聚丙烯盖。抬起铝圈。拔下金属密封圈。取下银环。
移去橡胶封口。在无菌条件下,吸取所需体积的Ficoll-Paque培养基(图2)。 - 加入Ficoll-Paque培养基(3 mL)至离心管中。
- 小心将稀释的血液样品(4 mL)铺在Ficoll-Paque培养基溶液上(图3)。
重要提示:在铺垫样品时不要将Ficoll-Paque培养基和稀释的血液样品进行混合。 - 18ºC至20°C条件下400 g离心30至40分钟(应当关闭制动)。
- 使用无菌移液管抽出含有血浆和血小板的上层,使单核细胞层在界面处不受干扰(图4和图5)。可将含有血浆的上层进行保存以供以后使用。
- 用无菌移液管将单核细胞层转移至一支无菌离心管中。

图 1.聚丙烯盖及银环的去除
图 2.Ficoll-Paque培养基的吸取
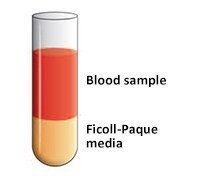
图 3.将血液样品铺在Ficoll-Paque培养基上
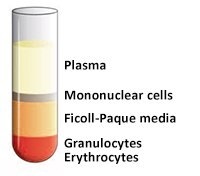
图 4.移除上层前。
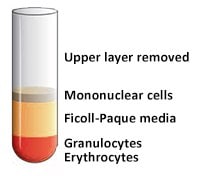
图 5.上层(血浆)移除后。
洗涤分离细胞
- 预估转移的单核细胞体积。加入至少3倍体积(~6 mL)的平衡盐溶液至离心管中的单核细胞中。
- 通过轻轻上下吹打将细胞进行悬浮。
- 18ºC至20°C条件下400-500 g离心10-15分钟。
注意:高速离心会提高单核细胞的回收率。然而,如果去除血小板也很重要,则推荐更低的离心转速(60-100 x g)。
- 去除上清液。
- 在6-8 ml的平衡盐溶液重悬单核细胞。
- 18ºC至20°C条件下400 -500 x g(或60-100 x g以去除血小板)离心10分钟。
- 去除上清液。
- 针对应用用适当的培养基重悬细胞沉淀。
粒细胞分离操作步骤
- 按照单核细胞分离操作步骤第1到6步利用Ficoll-Paque培养基进行密度梯度离心。
- 用无菌移液器将上层的Ficoll-Paque培养基吸出,留下位于红细胞层上的粒细胞白细胞层并确保其不被搅乱。
- 利用移液器收集较薄的粒细胞白细胞层,将其转移至无菌的离心管中。
- 用至少五倍体积的平衡盐溶液将细胞进行重悬并400 x g离心15分钟。
- 用任何可选的红细胞裂解液将剩余的红细胞进行裂解。
- 18ºC至20°C条件下400-500 x g离心10-15分钟。
- 去除上清液。
- 用6至8 ml的平衡盐溶液重悬粒细胞。
- 18ºC至20°C条件下400-500 x g离心10分钟。
- 去除上清液。
- 针对应用用适当的培养基重悬细胞沉淀。
注
抗凝剂:肝素、EDTA、柠檬酸盐、酸性柠檬酸葡萄糖(ACD)和柠檬酸盐磷酸盐葡萄糖(CPD)均可用作血液样品的抗凝剂。去纤维血液不需要抗凝血剂。但去纤维化导致单核细胞产量降低,并可能增加红细胞的污染3。还会导致单核细胞的选择性丧失。
Bøyum发现使用EDTA代替肝素作为抗凝剂可获得略微更纯的单核细胞制品3。在从外周血以外的样品进行单核细胞纯化时也发现添加肝素可能会导致细胞悬浮液的凝胶化4。柠檬酸盐稳定的血液可以产生比其他抗凝剂更高质量的RNA和DNA,并产生更高的单核细胞产量。肝素会影响T细胞增殖并与许多蛋白发生结合。EDTA适用于基于DNA的检测,但会影响Mg2+浓度并对细胞遗传学分析产生影响EDTA5。血液中的RNA产量也显示高于肝素血液6。
血液体积:通过使用更大直径的离心管并保持与上述标准方法大致相同的Ficoll-Paque培养基(2.4cm)和血液样品(3.0cm)的高度,可以实现以相同的分离效率处理更大体积的血液。增加管直径不会影响所需的分离时间。
血液样品储存:血液样品应无凝块并在收集后尽快处理以确保获得最佳的结果。延迟处理血液可导致活力丧失、细胞回收率降低以及更多的粒细胞或红细胞污染。实际上,有报道称在室温下储存24小时会导致淋巴细胞产量降低、表面标志物表达改变以及对促有丝分裂刺激的反应降低5,7,8。去除死细胞:使用Ficoll-Paque PLUS在细胞分离过程中去除死细胞9,10,58。
密度和温度:进行密度梯度分离时的温度自然会受室温、离心温度、密度梯度介质温度和液体样品温度的影响。对于室温的含义,有时也会产生混淆(例如,在欧洲它可能是20ºC而在北美则是>20ºC)。众所周知,Ficoll-Paque产品的密度随着温度的升高而降低。例如,Ficoll-Paque PREMIUM (1.077 g/ml)在20ºC、22ºC和25ºC时密度分别是1.0772、1.0767和1.0758。
病理性血液样本:上述标准方法已被开发用于从正常健康的人类供体的外周血中纯化单核细胞。取自具有感染或其他病理状况,如癌症的供体样品可能会获得不同的结果(参考Ficoll-Paque PLUS及Ficoll-Paque PREMIUM的进一步应用,第12页)。
血小板去除:如果从单核细胞组分中除去除所有血小板是一项重要的要求,则可在Ficoll-Paque PLUS之上铺垫4%至20%蔗糖梯度并进行二次离心。该过程将有效去除任何的血小板污染11。血小板将保留在蔗糖梯度的顶部,并且单核细胞可通过蔗糖梯度沉淀到Ficoll-Paque PLUS层的顶部。也可在分离单核细胞之前通过与腺苷-5'-二磷酸盐(ADP)聚集来去除血小板12。
效果不佳时的问题排除
如果按照推荐的标准过程进行使用,能够预期Ficoll-Paque PLUS和Ficoll-Paque PREMIUM可顺利从人外周血中进行单核细胞分离,相应的结果如第2页所示。如前所述,Ficoll-Paque PREMIUM 1.073和Ficoll-Paque PREMIUM 1.084将得到略微不同单核细胞密度群体的细胞制品。然而,某些实验参数的偏差可能也会导致结果不佳,这里给出的问题排除图表旨在帮助快速识别并纠正导致性能下降的问题。
材料
参考文献
如要继续阅读,请登录或创建帐户。
暂无帐户?