Sepharose XL – for Selected Proteins that Require Very High Binding Capacity to Increase Productivity, Easy Scale-up
Use Sepharose XL media for purification of proteins when improved binding capacity compared to other Sepharose media has been confirmed for the selected protein.
Use Sepharose XL at the beginning of a purification scheme for initial capture when a high binding capacity and rapid separation is required for a selected protein from clarified samples.
Run columns packed with Sepharose XL on systems such as ÄKTAdesign, FPLC System and HPLC or systems using peristaltic pumps. Appendix 4 gives guidance on how to select the most suitable ÄKTAdesign system.
Purify viruses or viral vectors using Q Sepharose XL virus licensed.
Sepharose XL media are based on a matrix of 90 μm particles, made from 6% agarose and highly cross-linked for chemical and physical stability, substituted with quaternary ammonium (Q) or sulfopropyl (SP) groups. The ionic groups are bound to long, flexible dextran chains which have been coupled to the agarose. This increases the exposure of the Q or SP groups thereby raising the binding capacity to a very high level without restricting the passage of charged molecules. The strong ion exchange groups maintain their charge over a broad pH range, allowing selection of the most suitable pH for each application. Particle size and bed volumes remain stable, despite changes in ionic strength or pH, to ensure fast separations at high flow rates with good resolution.
Purification options

Figure 62. Q and S Sepharose XL are available in prepacked HiTrap and HiPrep columns, in media packs and in the Ion Exchange Selection Kit.
*Appendix 5 to convert linear flow (cm/hour) to volumetric flow rates (mL/min) and vice versa.
**Working pH range refers to the pH interval where the medium binds protein as intended or as needed for elution without adverse long term effects.
***Maximum operating back pressure refers to the pressure above which the medium begins to compress.
**** Important information: Separating viral particles with Q Sepharose XL may require a license under United States patent 6,537,793 B2 and foreign equivalents owned by Gencell SAS. Such a license is not included with the purchase of Q Sepharose XL, but is included with the purchase of Q Sepharose XL virus licensed products. Purchasers of Q Sepharose XL virus licensed products are granted a free limited license under US patent 6,537,793 B2 and foreign equivalents owned by Gencell SAS to separate viral particles solely through use of the product purchased.
Use prepacked HiTrap columns (1 mL or 5 mL) for media selection, method scouting, group separations, small scale purification, sample concentration or clean-up. Connect up to 3 HiTrap columns in series to scale-up.
Use prepacked HiPrep columns (20 mL) for method development, group separations, larger scale purification, sample concentration or clean-up. Connect several HiPrep columns in series to increase binding capacity.
For column packing:
Purification examples
Media selection
Using 1 mL HiTrap columns the most suitable matrix and charged group for a separation to be selected quickly and easily. In Figure 63 a comparison of elution profiles for the same sample separated under identical conditions on three different media illustrates the differences in selectivity and resolution that can result from changing the charge group and matrix. The most suitable medium can be selected and conditions optimized according to the requirements for the purification. In this example Sepharose XL resolves the three components and optimization of elution conditions could further improve the resolution. However, any of these media would be suitable if the aim was to isolate the first major peak (ribonuclease A).
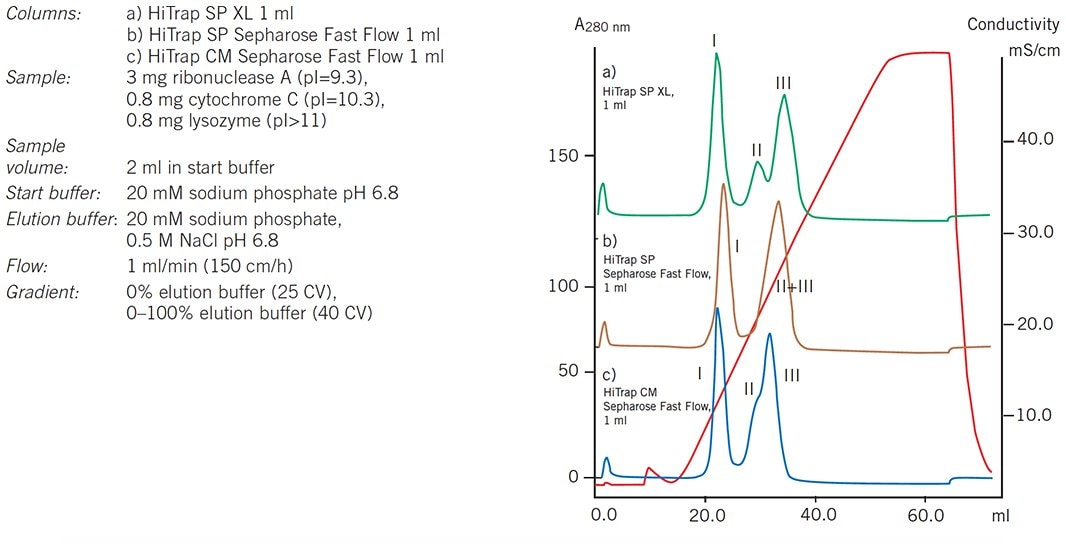
Figure 63. Media scouting: separation of ribonuclease A (I), cytochrome c (II) and lysozyme (III) on a range of anion exchange HiTrap columns.
Capture
Capture of alkaline phosphatase from a clarified lysate of E. coli using a HiTrap Q XL 1 mL column is shown in Figure 64. Separation was monitored at A280 nm and phosphatase activity assayed by a spectrophotometric method at A405 nm
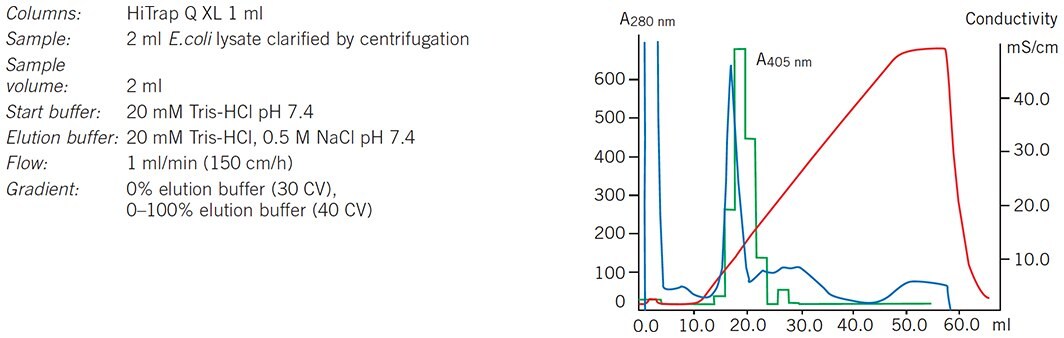
Figure 64. Clarified E. coli lysate on HiTrap Q XL, absorbance values at 450 nm relate to phosphatase activity in eluted fractions.
Capture and Scale-up
Figure 65 shows a pilot scale purification performed on a Sepharose XL ion exchanger. The separation was developed on Q Sepharose XL packed in an XK 16/20 column in order to select optimal pH and to determine maximum binding capacity available. Adding CaCl2 to the sample precipitated DNA and so increased the binding capacity for the target protein. Final loading was reduced to 75% of the maximum capacity and the result verified before scaling-up to the larger column.
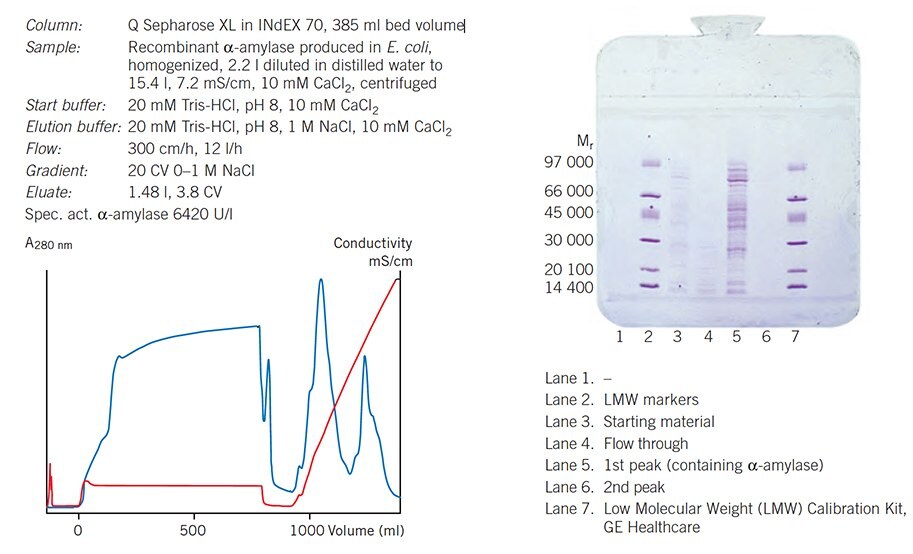
Figure 65. Capture and scale-up A pilot scale purification performed on a Sepharose XL ion exchanger. The separation was developed on Q Sepharose XL packed in an XK 16/20 column in order to select optimal pH and to determine maximum binding capacity available. Adding CaCl2 to the sample precipitated DNA and so increased the binding capacity for the target protein. Final loading was reduced to 75% of the maximum capacity and the result verified before scaling-up to the larger column.
Viral Purification
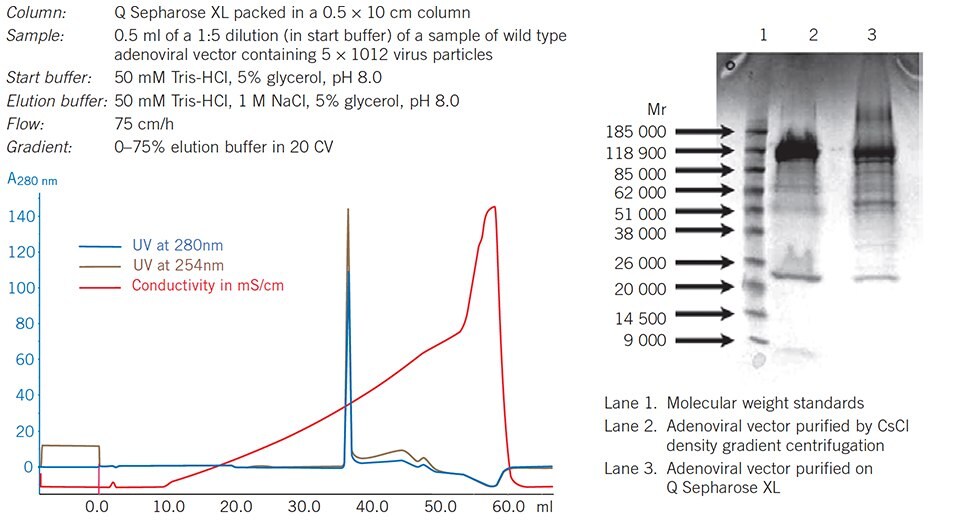
Figure 66. Q Sepharose XL virus licensed provides an alternate to traditional cesium chloride density gradient centrifugation used for purification of viruses and viral vectors.
Sample Concentration
It can be an advantage to concentrate a sample prior to gel filtration in order to minimize sample volume and facilitate a rapid, high resolution size separation. HiTrap columns offer a convenient, ready to use solution for sample concentration. Table 7 on page 89 gives examples of the high concentration factors achieved when concentrating proteins from very dilute starting material using HiTrap columns prepacked with Sepharose HP medium. Similar results can be achieved with HiTrap columns prepacked with Sepharose Fast Flow or Sepharose XL media.
Performing a separation
Guidelines for selection of media, buffer, pH and ionic strength conditions and method optimization are given in Chapter 2. Use the instructions given here as a basis from which to optimize a separation.
Correct sample and buffer preparation is essential in order to achieve optimal separation and avoid any deterioration in column performance. Samples must be fully dissolved and free from particles or other material likely to interfere with the separation. Refer to Chapter 2 and Appendix 1 for recommendations and advice on sample preparation.
Filter buffers after all salts and additives have been included. Use high quality water and chemicals. Filter solutions using filters of 1 μm or smaller. To avoid formation of air bubbles in a packed column, maintain buffers and columns at a constant temperature before and during a run.
The pH of the start buffer should be at least 0.5–1 pH unit above the pI of the target substance when using an anion exchanger (Q) and 0.5–1 pH unit below the pI of the target substance when using a cation exchanger (S). See Appendix 2 for recommendations on volatile and non-volatile buffer systems for anion and cation exchangers.
For samples with unknown charge properties, try the following:
- anion exchange (Q)
start buffer: pH 8.0
elution buffer: start buffer including 1 M NaCl, pH 8.0 - cation exchange (SP)
start buffer: pH 6.0
elution buffer: start buffer including 1 M NaCl, pH 6.0
Users of ÄKTAdesign systems with BufferPrep functionality can select one of the buffer recipes recommended for anion exchange chromatography at pH 8 or cation exchange chromatography at pH 6.
First time use or after long term storage
- To remove ethanol, wash with 1 column volume of distilled water at 1 mL/min (HiTrap 1 mL), 5 mL/min (HiTrap 5 mL), 5 mL/min (HiPrep 20 mL), or at 50 cm/h for Sepharose XL packed in larger columns. This step ensures removal of ethanol and avoids the risk of precipitation if buffer salts were to come into contact with the ethanol. The step can be omitted if precipitation is not likely to be a problem.
- Wash with 5 column volumes of start buffer at 1 mL/min (HiTrap 1 mL), 5 mL/min (HiTrap 5 mL), 5 mL/min (HiPrep 20 mL) or at 150 cm/h for Sepharose XL packed in larger columns.
- Wash with 5 column volumes of elution buffer, same flow as step 2.
- Wash with 5 column volumes of start buffer, same flow as step 2.
- Run a blank elution before applying sample.
Separation by gradient elution
Flow: 1 mL/min (HiTrap 1 mL), 5 mL/min (HiTrap 5 mL), 5 mL/min (HiPrep 20 mL) or at 150 cm/h for Sepharose XL packed in larger columns. Collect fractions throughout the separation.
- Equilibrate column with 5–10 column volumes of start buffer or until the baseline, eluent pH and conductivity are stable.
- Adjust the sample to the chosen starting pH and ionic strength and apply to the column.
- Wash with 5–10 column volumes of start buffer or until the baseline, eluent pH and conductivity are stable i.e. when all unbound material has washed through the column.
- Begin elution using a gradient volume of 10–20 column volumes and an increasing ionic strength up to 0.5 M NaCl (50%B).
- Wash with 5 column volumes of 1 M NaCl (100%B) to elute any remaining ionically-bound material.
- Re-equilibrate 5–10 column volumes of start buffer or until eluent pH and conductivity reach the required values.
Separation by step elution
Flow: 1 mL/min (HiTrap 1 mL), 5 mL/min (HiTrap 5 mL), 5 mL/min (HiPrep 20 mL) or at 150 cm/h for Sepharose XL packed in larger columns.
Collect fractions throughout the separation.
- Equilibrate column with 5–10 column volumes of start buffer or until the baseline, eluent pH and conductivity are stable.
- Adjust the sample to the chosen starting pH and ionic strength and apply to the column.
- Wash with 5–10 column volumes of start buffer or until the baseline, eluent pH and conductivity are stable i.e. when all unbound material has washed through the column.
- Elute with 5 column volumes of start buffer + NaCl at chosen ionic strength.
- Repeat step 4 at higher ionic strengths until the target protein(s) has been eluted.
- Wash with 5 column volumes of a high salt solution (1 M NaCl in start buffer) to elute any remaining ionically bound material.
- Re-equilibrate with 5–10 column volumes of start buffer or until eluent pH and conductivity reach the required values.
Save time by using higher flow rates during the high salt wash and re-equilibration steps.
Do not exceed the maximum recommended flow for the medium.
If ionic detergents have been used, wash the column with 5 column volumes of distilled water, followed by 2 column volumes 2 M NaCl. Re-equilibrate with at least 10 column volumes of start buffer until the UV baseline, eluent pH and/or conductivity are stable.
Organic solvents such as ethanol can be used to remove non-ionic detergents. When selecting an organic solvent, check the chemical stability of the medium to determine a suitable concentration.
Check column performance regularly by determining column efficiency and peak symmetry.
See Appendix 3.
Cleaning
Correct preparation of samples and buffers and application of a high salt wash (1 M NaCl) at the end of each separation should keep most columns in good condition. However, reduced performance, a slow flow rate, increasing back pressure or complete blockage are all indications that the medium needs to be cleaned using more stringent procedures in order to remove contaminants.
It is recommended to reverse the direction of flow during column cleaning so that contaminants do not need to pass through the entire length of the column. The number of column volumes and time required for each cleaning step may vary according to the degree of contamination. If the cleaning procedure to remove common contaminants does not restore column performance, change the top filter (when possible) before trying alternative cleaning methods. Care should be taken when changing a filter as this may affect the column packing and interfere with performance.
The following procedure should be satisfactory to remove common contaminants:
- Wash with at least 2 column volumes of 2 M NaCl at 1 mL/min (HiTrap 1 mL), 5 mL/min (HiTrap 5 mL), 5 mL/min (HiPrep 20 mL) or at 40 cm/h with a contact time of 1–2 hours for Sepharose XL packed in larger columns.
- Wash with at least 4 column volumes of 1 M NaOH (same flow as step 1).
- Wash with at least 2 column volumes of 2 M NaCl (same flow as step 1).
- Rinse with at least 2 column volumes of distilled water (same flow as step 1) until the UV-baseline and the eluent pH are stable.
- Wash with at least 4 column volumes of start buffer or storage buffer (same flow as step 1) until eluent pH and conductivity have reached the required values.
To remove precipitated proteins, lipids, hydrophobically bound proteins or lipoproteins, refer to Appendix 1.
Media Characteristics
Composition: sulfopropyl (SP) or quaternary amino (Q) groups attached via chemically stable ether bonds to long, flexible dextran chains that are covalently coupled to highly cross-linked 6% agarose.
*Long term pH stability refers to the pH interval where the medium is stable over a long period of time without adverse side effects on the chromatography performance.
Short term pH stability refers to the pH interval for regeneration, cleaning-in-place and sanitization procedures.
All ranges are estimates based on the experience and knowledge within Cytiva.
Chemical stability
For daily use, Sepharose XL media are stable in all common, aqueous buffers, 1 M NaOH, denaturing agents (8 M urea, 6 M guanidine hydrochloride), with additives such as non-ionic detergents, 70% ethanol, 1 M acetic acid and 30% isopropanol.
Sepharose XL can be used with organic solvents such as dimethylsulfoxide, dimethylformamide, tetrahydrofuran, acetone, chloroform, dichloromethane, dichloroethane and dichloroethane/pyridine (50:50) as well as polar solvents and aqueous/organic isolutions. The water in the medium can be exchanged by the alternative solvent with very little effect on the pore size of the matrix.
Avoid cationic detergents with SP Sepharose XL. Avoid anionic detergents with Q Sepharose XL. Avoid oxidizing agents.
Storage
For column storage, wash with 2 column volumes of distilled water followed by 2 column volumes of 20% ethanol. Include 0.2 M sodium acetate in the storage solution for SP Sepharose XL. Degas the ethanol/water mixture thoroughly and apply at a low flow rate to avoid over-pressuring the column. Store at room temperature or, for long periods, store at +4 °C to +8 °C. Ensure that the column is sealed well to avoid drying out. Whenever possible, use the storage and shipping device if supplied by the manufacturer. Store unused media at +4 °C to +30 °C in 20% ethanol. Do not freeze.
To avoid formation of air bubbles in a packed column, ensure that column and buffers are at the same temperature when preparing for a run.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?