Fast Vitamin D Metabolite Analysis
Introduction
- Vitamin D deficiency has become a topic of interest in recent publications. Vitamin D regulates the absorption of calcium within the digestive track and is important to promote proper bone growth and prevent osteoprosis in older adults.
- This past year, the Institute of Medicine has increased the recommended daily value of vitamin D.
- Vitamin D is present in two forms, Vitamin D3 and Vitamin D2. Vitamin D3 is produced in the skin after exposure to ultraviolet B light from the sun and other artificial sources.
- Vitamin D3 along with Vitamin D2 (ergocalciferol) are used to fortify various foods and over-the-counter supplements.
- Immunoassay methods for Vitamin D can suffer from inaccuracy due to protein binding effects. Thus, analytical methods that can accurately quantitate both 25-hydroxyvitamin D forms are essential for diagnosis and monitoring patients with vitamin D disorders.
- Recent studies have indicated that separation from the inactive 3-epi analogs may provide more accurate information for treatment and prevention.
- Testing for Vitamin D metabolites in humans cannot distinguish D2 and D3 forms of the vitamin, resulting in a reporting of only total 25- hydroxyvitamin D. Furthermore, both forms are metabolized to their respective 25-hydroxyvitamin D form.
Vitamin D Sources
- In this study the effects of chromatographic selectivity along with sample preparation techniques are evaluated for the efficient quantitation of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and epi-25-hydroxyvitamin D3
- Published HPLC methods for the analysis of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 depict the use of C18 or Cyano stationary phases.
- Resolution can be achieved between 25-hydroxyvitamin D3 and epi-25-hydroxyvitamin D3 on Cyano phases but at the expense of long elution times.
- The goal was to evaluate different HPLC phase chemistries for resolution of metabolites with minimal analysis time. The use of both UV and MS detection was employed for method development.
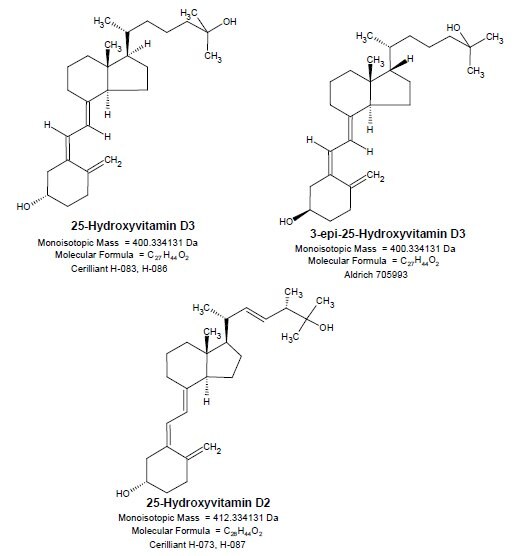
Structures
Experimental
- In the first part of this study, chromatographic selectivity was evaluated using C18 and F5 stationary phases with standard solutions. Analyte monitoring was conducted using both UV and MS detection methods.
- The goal was to develop fast chromatographic conditions; <5 minute run time.
- Figure 1 depicts the analysis of 25-hydroxyvitamin D3, epi-25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 on a traditional C18 stationary phase.
- 25-hydroxyvitamin D3 can be resolved from 25-hydroxyvitamin D2 but coelution occurs between 25-hydroxyvitamin D3 and epi-25-hydroxyvitamin D3.
- Analysis on C18 phases causes further problems for LC-MS/MS applications, because 25-hydroxyvitamin D3 and epi-25-hydroxyvitamin D3 are isobaric, thus preventing accurate quantitation.
- Figure 2 demonstrates the Ascentis Express F5 phase for resolution of 25-hydroxyvitamin D3, epi-25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in less than four minutes, enabling quantitation of all three components in one chromatographic analysis.
- LC-MS analysis methodologies for 25-hydroxyvitamin D3 are not without disadvantages also.
- Many assay samples are derived from serum/plasma. The second portion of the study was to focus on the impact of the sample preparation technique on analyte response.
- Sample preparation from serum requires protein precipitation with organic solvents or strong acids. This technique results in gross depletion levels of proteins from the sample, but also results in high levels of matrix interference from the co-extracted phospholipids.
- The co-extracted phospholipids can result in quantitative irregularities and decrease sample throughput due to gradient column washing requirements.
- In this study, the HybridSPE®-Phospholipid was utilized to selectively extract the phospholipids from the serum sample. This technique has the simplicity of performing standard protein precipitation but with the added benefit of additional matrix removal.
- All serum samples were assayed on the Ascentis Express F5 developed method.
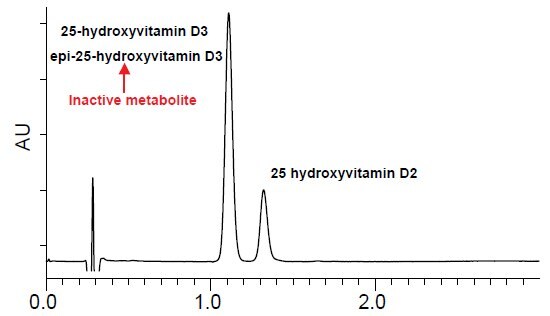
Figure 1.Vitamin D Metabolites on Ascentis® Express C18
Retention Time:
25-hydroxyvitamin D3 1.09 min.
epi-25-hydroxyvitamin D3 1.09 min.
25-hydroxyvitamin D2 1.29 min.
column: Ascentis Express C18, 5 cm x 2.1 mm, 2.7 μm (53822-U)
mobile phase A: 15%, 5 mM ammonium formate water
mobile phase B: 85%, 5 mM ammonium formate (95:5 acetonitrile:water)
flow rate: 0.4 mL/min.
temp.: 35 °C
det.: UV 265 nm, MS ESI+ 100-1000m/z
injection: 1 μL
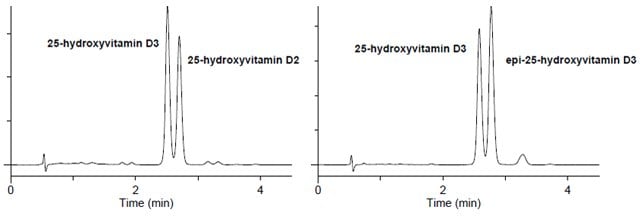
Figure 2. Vitamin D Metabolites on Ascentis Express F5
Retention Time:
25-hydroxyvitamin D3 2.57 min.
epi-25-hydroxyvitamin D3 2.76 min.
25-hydroxyvitamin D2 2.77 min.
column: Ascentis Express F5, 10 cm x 2.1 mm, 2.7 μm (53569-U)
mobile phase A: 25%, 5 mM ammonium formate water
mobile phase B: 75%, 5 mM ammonium formate (methanol)
flow rate: 0.4 mL/min.
temp.: 40 °C
det.: UV 265 nm, MS ESI+ 100-1000m/z
injection: 1 μL
- Table 1 summarizes the difference and detected analytes using from plasma, the two different HPLC phase chemistries.
- The coelution of the isobaric compounds on the C18 resulted in a arbitrarily high detected level of the 25-hydroxyvitamin D2.
- The selectivity of the F5 phase enabled resolution of the isobaric compounds resulting in more accurate measurement.
Sample Preparation
Wellplate: HybridSPE-Phospholipid 50 mg 96-well plate (575656U)
Rat serum was spiked at 300 ng/mL with 25-hydroxyvitamin D2, 25- hydroxyvitamin D3 and epi-25-hydroxyvitamin D3. Protein precipitation was performed offline by adding 100 μL of spiked serum into a 500 μL 96-well collection plate followed by 300 μL of 1% formic acid acetonitrile. Samples were mixed by performing five 300 μL draw/aspiration cycles using a digital pipetter, then allow to set for 5 minutes before transferring 200 μL of precipitate into the HybridSPE-Phospholipid 96-well plate. Samples were passed through the HybridSPE-Phospholipid plate by applying 10” Hg vacuum for 4 minutes, the resulting filtrate was analyzed directly.
As a comparison, spiked rat serum was also processed using standard protein precipitation by adding 100 μL of serum into 2 mL centrifuge vials followed by 300 μL of 1% formic acid in acetonitrile. Samples were vortexed and centrifuged, and the resulting supernatant was collected and analyzed directly.
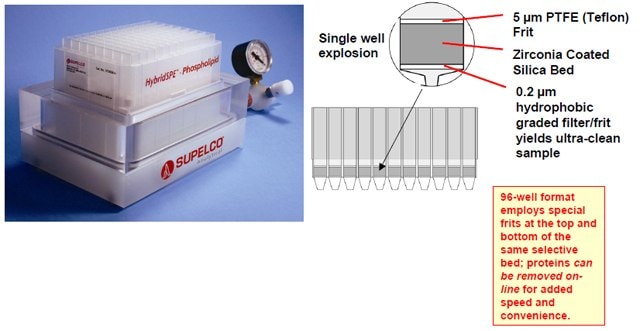
Figure 3. Sample process for HybridSPEPhospholipid 96-well Plate
- The selective extraction of phospholipids is achieved using a novel zirconia-coated particle technology.
- The high selectivity towards phospholipids is achieved utilizing Lewis acid/base interaction between the phosphate group of the phospholipids and the zirconia surface.
- The zirconia-coated particle is not as Lewis “acidic” as pure zirconium oxide, thus enabling highly efficient extraction of phospholipids while remaining non-selective towards a broad range of basic, neutral and acidic compounds.
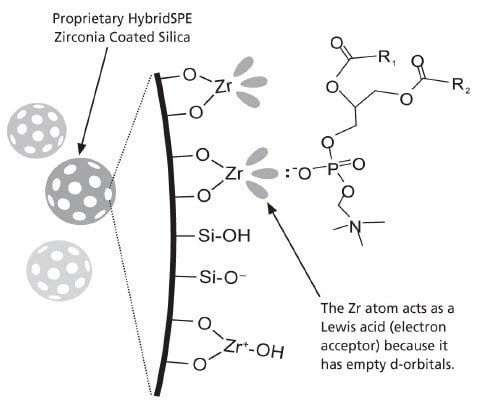
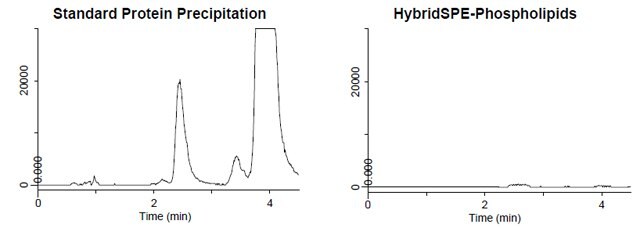
Figure 4. Sample Preparation Comparison: Phospholipid Monitoring
Phospholipid Monitoring (m/z):
Lysophosphatidylcholines: 496.3, 524.3 m/z
Glycerophosphocholines: 758.5, 786.5, 806.5, 810.5 m/z
instrument: Agilent 1200 SL Rapid Resolution, 6210 TOF
column: Ascentis Express F5, 10 cm x 2.1 mm, 2.7 μm (53569-U)
mobile phase A: 25%, 5mM ammonium formate water
mobile phase B: 75%, 5mM ammonium formate methanol
flow rate: 0.4 mL/min
temp.: 40 °C
MS det.: 100-1000m/z
Cap V: 3000V, Fragmenter: 200V, Dry Gas: 10L/min, Gas Temp: 350 °C
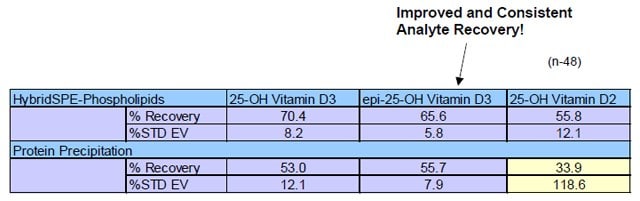
Table 2. Sample Prep Analyte Recovery Comparison from Spiked Rat Serum
Sample spike level of 300 ng/mL, n=48 replicates, external calibration
Results
- Figure 4 depicts the phospholipid monitoring chromatograms of coextracted matrix from standard protein precipitation and using the HybridSPE-Phospholipids technique.
- The HybridSPE-Phospholipids technique selectively depleted the phospholipid matrix resulting in no matrix interference.
- The standard protein precipitation extracted contained a large amount of co-extracted phospholipid matrix is resulting in interference that co-elutes directly in the elution range of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and epi-25-hydroxyvitamin D3.
- This co-elution has the potential to cause sensitivity and reproducibility issues resulting in irregularities in quantitation.
- Table 2 depicts the analyte recovery using the standard protein precipitation technique ranged from 33% to 55% with very high variability for 25-hydroxyvitamin D2.
- Higher and more consistent recovery was observed using the HybridSPEPhospholipids technique.
Summary
- Chromatographic resolution of analytes still plays an important role in LC/MS applications when dealing with isobaric compounds.
- The unique selectivity of the Ascentis Express F5 enables a fast and efficient method for the analysis of 25-hydroxyvitamin D and related forms from serum samples.
- The selective phospholipid depletion of the HybridSPE-Phospholipid 96-well plate enable an efficient sample cleanup increasing method reproducibility and accuracy.
- This approach demonstrates how selectivity, in both chromatographic and sample preparation, allows for efficient analysis that would otherwise be unattainable with traditional reversed phase approaches.
- The combination of this novel sample prep technique along with the unique selectivity of the Ascentis Express F5 enables a fast and simplified bioanalytical method for associated Vitamin D metabolites.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?