CBILS©: Cell-Based Immunotherapy Logistics System
A Revolutionary Tool for Ionic Liquid Synthesis
The use of CBILS© – Carbonate Based Ionic Liquid Synthesis – developed by the Austrian company proionic, offers one of the simplest, yet most elegant synthetic methods for the production of ionic liquids.1 There are numerous methods for syntheses of ionic liquids existing, e.g. by alkylation of tertiary amines, reaction of a Lewis acid with a quaternary ammonium salt, anion exchange of a quaternary ammonium salt via metathesis etc.2 which has finally led to a fantastic amount of new literature in the past years.3
Now, with CBILS© it is possible to synthesize 20 or more new ionic liquids per day in a modular and systematic way!
How Does CBILS© Work?
CBILS© are composed of a nitrogen or phosphorus cation (e. g. 1-ethyl-3-methylimidazolium) and a negatively charged counterpart of a hydrogencarbonate or methylcarbonate anion respectively. When CBILS© are treated with virtually any Brønsted acid of your choice the carbonate anion moiety is decomposed to one equivalent of H2O or MeOH and one equivalent of gaseous CO2 (Scheme 1).
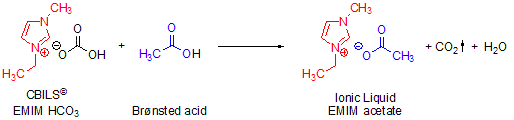
Scheme 1.Straightforward synthesis of 1-ethyl-3-methylimidazolium acetate using CBILS© EMIM HCO3
In these exchange reactions, the chemical equilibrium is shifted continuously by the formation of gaseous carbon dioxide; therefore the reaction always works quantitatively with every Brønsted acid available, even very insoluble ones. The CO2 is ultimately removed from the reaction and in the same step replaced by the anion of the Brønsted acid used. The desired ionic liquid is then easily isolated by evaporative removal of the reaction by-product.
Choose your anion out of thousands of commercially available Brønsted acids – from simple mineral acids to the most complicated, chiral and functionalized acids, from strong to weak ones, and from soluble to nearly insoluble ones. You can also use Brønsted acid precursors such as organic or inorganic anhydrides. For example, it was possible to successfully synthesize ionic liquid molybdates and tungstates using molybdenum oxide and tungsten oxide respectively, both of which are very insoluble in aqueous media. Although sluggish, both ionic liquids were formed at 100% conversion as evidenced by the slow, but continuous evolution of CO2.
Details about CBILS©
Calculating the exact reaction stoichiometry is critical, because the ionic liquid may be sensitive to unreacted free acid or carbonate precursor. The concentration of CBILS© precursors is indicated on the label and is lot specific. The concentration of the acid you are using must be determined via the certificate of analysis or alkalimetric titration and should be known to within 1% accuracy. By changing the stoichiometry of a diprotic Brønsted acid, you can synthesize hydrogenated anions with ease (Scheme 2):
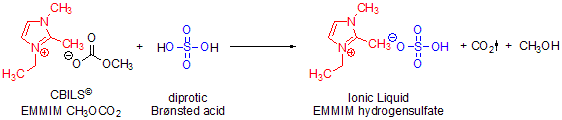
Scheme 2.Formal synthesis of 1-ethyl-2,3-dimethylimidazolium hydrogensulfate using CBILS© EMMIM CH3OCO2 and sulfuric acid as diprotic Brønsted acid
Some anions are not accessible via Brønsted acids because the free acid is not stable or not known at all. A typical example is the thiocyanate anion, SCN–. Free thiocyanic acid (HSCN) is only stable at 0 °C in 5% aqueous solution and is not commercially available. In this case, you can use the corresponding ammonium salt, gently heating the reaction mixture to 60 °C for at least 30 minutes and removing the solvents at a temperature of at least 70 °C (Scheme 3):
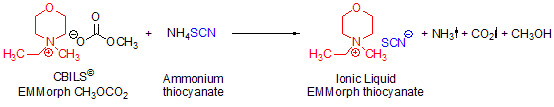
Scheme 3.Synthesis of N-ethyl-N-methylmorpholinium thiocyanate using CBILS© EMMorph CH3OCO2 and ammonium thiocyanate as stable equivalent to the not directly accessible thiocyanic acid (HSCN)
A second alternative to Brønsted acids is the reaction of a CBILS© carbonate precursor with a calcium, zinc, manganese or other metal salt, which results in the formation of an insoluble metal carbonate. For example, if you react calcium thiocyanate (Ca(SCN)2) with a carbonate precursor, the desired thiocyanate based ionic liquid is formed and calcium carbonate precipitates. The resultant solid can easily be removed by filtration or centrifugation (Scheme 4):
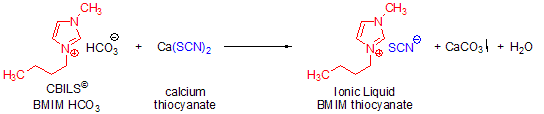
Scheme 4.Synthesis of 1-butyl-3-methylimidazolium thiocyanate using CBILS BMIM HCO3 and calcium thiocyanate.
One Hundred Syntheses in One Week?
By using the CBILS© route for the synthesis of ionic liquids and analogous structures, it is possible for a single person, in one week’s time, to synthesize 100 new substances via the combination of 10 precursors with 10 Brønsted acids. There is no need for the use of dry solvents, no halogen impurities or by-products are formed, no guesswork about the synthetic route, and no waste is generated. Just select the cation precursor and the anion (via the acid) of your choice, and make your dream ionic liquid a reality!
In summary to synthesize your desired ionic liquid all you simply need to do is:
- Choose the cation in the form of the corresponding CBILS© precursor.
- Choose the anion in the form of the corresponding Brønsted acid.
- Calculate the necessary stoichiometry to form the ionic liquid.
- Mix the CBILS© precursor and the Brønsted acid and stir until CO2 generation subsides – depending on the batch and the substance, this typically requires 5 minutes to 1 hour.
- Remove the solvent and reaction by-product (water or methanol) by rotary evaporation.
We now offer a selection of eleven CBILS© precursors for the synthesis of imidazolium, ammonium, phosphonium, pyrrolidinium, piperidinium, and morpholinium type ionic liquids in the form of their methylcarbonates and hydrogencarbonates. All CBILS© ionic liquid precursors are delivered in 40 to 60% aqueous or aqueous/methanolic solutions.
References:
如要继续阅读,请登录或创建帐户。
暂无帐户?