QuinoxP*
Introduction
Various optically active phosphine ligands incorporating a chiral center at phosphorus display exceptional enantiosectivities in metal-catalyzed asymmetric synthesis.1 For instance, known classes P-chiral phosphine ligands offer good to excellent enantiocontrol in Ru- and Rh-catalyzed hydrogenation reactions.2 The one limitation associated with these ligands is their sensitivity to air, which has impeded widespread applicability in bench-top chemistry. Imamoto and co-workers have addressed this deficiency through the invention of QuinoxP*, which contains an electron-withdrawing quinoxaline architecture.3 In collaboration with Nippon Chemical, we are pleased to offer R,R-QuinoxP* for the research market.†
Advantages of the QuinoxP* Ligands:
- QuinoxP* is not oxidized nor epimerized at ambient conditions in air
- Enantioselectivities are outstanding for various reaction paradigms
- Hydrogenations proceed under mild reaction conditions
- Low catalyst loadings yield high TONs
Representative Applications:
The reactivity profile of these innovative, chiral ligands is covered below and highlights the impressive breadth of valuable transformations mediated by QuinoxP*. These powerful efficient ligands exhibit high levels of enantiocontrol in synthetic transformations ranging from metal-catalyzed asymmetric 1,4-additions of arylboronic acids, to enantioselective alkylative ring opening, to asymmetric hydrogenations.3 It is worth noting that QuinoxP* is not oxidized at the stereogenic phosphorus center on standing at ambient temperature in air for more than 9 months.
Highly Asymmetric Rhodium-Catalyzed Hydrogenation
Imamoto has gone to great lengths to develop enantiomerically pure P-chiral ligands for industrially useful transformations such as asymmetric hydrogenation. Impressively, a diverse array of prochiral amino acid and amine substrates were hydrogenated with great efficiency to yield highly enantiopure amine derivatives (Scheme 1). The authors carried out these experiments at room temperature in methanol under low hydrogen pressures (3 atm). Note that all hydrogenation reactions were complete in 6 hours and with enantiomeric excesses ranging from 96 to 99.9%. Dramatic stereochemical reversal, consistent with the results observed with the related (S,S)-tert-Bu-BisP* ligands,4,5 was obtained when 1-acetylamino-1-adamantylethene was hydrogenated to afford the S configuration amine with > 96% enantioselectivity (Table 1).
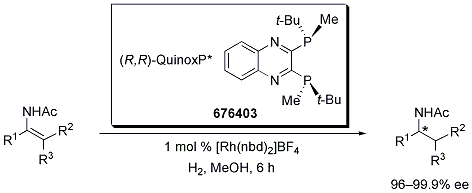
Scheme 1
Asymmetric 1,4-additions of Arylboronic Acids
Imamoto and co-workers exploited the high activity of the QuinoxP* ligand in rhodium-catalyzed enantioselective 1,4-additions of arylboronic acids to α,ß-unsaturated carbonyl substrates.3 High yields of the addition products were obtained via running the reactions between 40 and 50 ºC (Scheme 2). The exceptional enantiocontrol exerted by this Rh(I)-catalyzed system is evident when compared to the use of BINAP as the chiral ligand.6
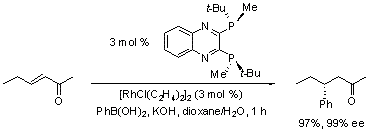
Scheme 2
Asymmetric Pd-catalyzed Ring Opening
Imamoto and co-workers have also succeeded in developing a Pd-catalyzed C-C bond-forming reaction, which displays high enantioselectivities with both dimethyl- and diethylzinc (Scheme 3, Table 2). This alkylative ring-opening methodology entails simply premixing PdCl2(cod) and QuinoxP* for 2 hours at room temperature - leading to a highly active catalyst. This catalyst system affords excellent yields of the ring-opened products and selectivities that rival the highest reported for this transformation. These results, when combined with the outstanding methodologies presented above, indicate that QuinoxP* is useful for a broad variety of asymmetric metal-catalyzed transformations.
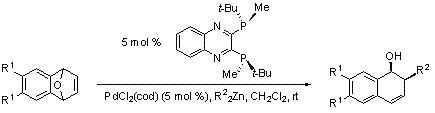
Scheme 3
Product Information
References
如要继续阅读,请登录或创建帐户。
暂无帐户?