Quantification of Psilocybin and Psilocin in “Magic Mushrooms”
Abstract
An LC-MS/MS method for quantifying psilocybin and psilocin in “magic mushrooms” was developed. The study reveals relative stability of these compounds up to room temperature, while elevated temperatures promote variable conversion and degradation, underscoring the need for standardized extraction protocols. Sample dilution might be required to prevent signal suppression.
Section Overview
Introduction
Tryptamines are a class of compounds that are known for their hallucinogenic effects and can be naturally found in plants, fungi, microbes, and amphibians. Tryptamines are structurally similar to serotonin, making them capable of binding to the serotonin receptor, thus causing psychedelic effects.1 Psilocybin mushrooms, or “magic mushrooms”, have been ingested for centuries due to their psychoactive properties, especially in spiritual ceremonies. Two components that are of particular interest are psilocybin and psilocin. Psilocybin, the inactive form, is very stable due to the phosphate group. Upon dephosphorylation, the compound becomes psilocin, which possesses hallucinogenic properties. In the past few decades, extensive research has been conducted to explore the potential therapeutic applications of these compounds in treating various mental disorders, including depression, anxiety, and post-traumatic stress disorder (PTSD). Recently some states have decriminalized the use of psychedelic mushrooms, while Oregon has made them fully legal2. Hence, accurate quantitation of psilocybin and psilocin as well as understanding appropriate dosing is crucial to ensuring the desired effects and minimizing potential risks.
In this study, we present a quantitation method for psilocybin and psilocin in “magic mushrooms”. Additionally, this method also detects structurally related compounds, such as aeruginascin, baeocystin, norbaeocystin, and norpsilocin. These compounds have been noted in the literature to complement the hallucinogenic properties of magic mushrooms.3 To assess the reliability of the method, bracketing injection and replicate sample analyses were employed, yielding RSDs of ≤5% for both psilocybin and psilocin in individual analyses. These results show good instrument and method suitability. The limit of quantitation (LOQ) achieved with this HPLC method was 1 ng/mL of injected extract. However, it is worth noting that detection was possible at an even lower concentration of 0.1 ng/mL.

Figure 1.Chemical structure and conversion of psilocybin to psilocin.
Experimental
Four strains of mushroom samples were separately cryo-milled into a powder. Samples included the canopy and stem portions of the fruiting bodies. To ensure reproducibility, triplicate measurements were taken for each mushroom strain, with a target weight of 50 mg of mushroom powder per assay. Psilocybin and psilocin were extracted by vortex shaking for 30 min at 2500 rpm in a total of 5 mL of 5% acetic acid in methanol. Following centrifugation, the resulting supernatant was transferred to a clean tube. Volume is brought to 10 mL with water and then further diluted 1000x with water. Diluted samples were spiked with an internal standard (Psilocin-D10 & Psilocybin-D4) for a final concentration of 50 ng/mL and then analyzed by LC-MS (Table 1). See below for a step-by-step overview of the sample preparation.
Extraction workflow steps for mushroom samples:
- CryoMill Mushrooms
- Weigh out 50 mg powder
- Add 3 mL 5% acetic acid in methanol
- Vortex for 30 minutes at 2500 rpm
- Centrifuge for 10 min at 4000 rpm
- Transfer supernatant to new tube
- Add 2 mL 5% acetic acid in methanol
- Vortex for 30 min at 2500 rpm
- Centrifuge for 10 min at 4000 rpm
- Transfer supernatant to existing supernatant
- Bring volume to 10 mL with water
- Dilute 1000x with water
- Spike with 50 ng/mL each Psilocin-D10 & Psilocybin-D4
- Submit for LC-MS/MS analysis (Table 1)
A calibration curve was created by preparing stock solutions of psilocybin and psilocin at 1.0 µg/mL in water and serially diluted to prepare the eight curve points, shown in Table 2. Each extracted sample was injected on column three times for a total of 9 injections per strain. The LOQ sample at 1 ng/mL was injected 6 times at the beginning of the run and 3 times at the end of the run to evaluate system suitability and drift.
Results
An 8-point, linear calibration curve was created for psilocybin and psilocin ranging from 1 ng/mL to 1000 ng/mL (Table 2) with weighting 1/x2. The curve was deemed acceptable if the correlation coefficient was greater than 0.99 and the accuracy of the calibration points was within 10% of the prepared concentration. Interestingly, the area response for psilocin was found to be 40-fold greater than psilocybin, despite the fact that analyzed psilocybin concentrations were much lower. Due to the high response, signal suppression of psilocin-D10 occurred when the native concentrations were 25 ng/mL and higher. Also, the accuracy of curve points 6 through 8 was not within the 10% acceptance criteria. Consequently, the last three curve points were dropped in the assessment of psilocin concentrations. Figure 2 illustrates the drop in internal standard area response with increasing native concentration in the calibration curve and shows increased sample response variability in samples that were more concentrated (samples diluted 100x versus samples diluted 1000x).

Figure 2.50 ng/mL Psilocin-D10 area response. Curve range 1 ng/mL to 1000 ng/mL. Samples diluted to 100x and 1000x.
Data displayed in Table 3 shows the concentration (% w/w) determined for psilocybin and psilocin for four mushroom strains. Each mushroom strain was weighed in triplicate and injected three times (n=9). The reported concentrations in Table 3 are an average of the 9 injections of 1000x diluted samples. This data was collected over three separate days, by two different analysts and two different LC-MS systems to evaluate the method for robustness. Unfortunately, what was not considered was within strain mushroom to mushroom variability. Literature shows that a large range of concentrations of psilocybin and psilocin can be present in mushrooms and may differ greatly depending on their cultivation, plant part, and storage conditions.5,6 Even under strict growing conditions the tryptamine content can vary widely within the same harvest.
During this study, the stability of cryo-milled mushrooms was assessed by subjecting them to different storage environments; this included a freezer, refrigerator, room temperature, and an elevated temperature of 40 C° with 75% relative humidity. The samples were packed in foil bags to protect them from air and light, then placed in the intended environment, and then analyzed over four weeks; the data obtained is presented in Table 4. Samples stored in the freezer served as the study’s control group. Upon analysis, it was found that while there is some variation in concentration for both psilocybin and psilocin when kept at refrigerated or room temperature conditions, there is a nearly 100% reduction in psilocybin when exposed to elevated temperatures. Psilocin, oppositely, exhibited varying rates of growth over the time course. These findings suggest that elevated temperatures have a profound effect on the conversion of psilocybin to psilocin. However, since this conversion is not one-to-one, we can speculate that there is degradation of psilocybin, psilocin, or both.
Other tryptamines present in psychedelic mushrooms include aeruginascin, baeocystin, norbaeocystin, and norpsilocin. The extent of the hallucinogenic properties and the ability to convert to a psychedelically active compound are still being studied. Having a single method that can easily examine the presence and/or concentration of all the tryptamines could be highly valuable. While only psilocybin and psilocin concentrations were evaluated, this method shows good separation of the other constituents, Table 5 and Figure 3.
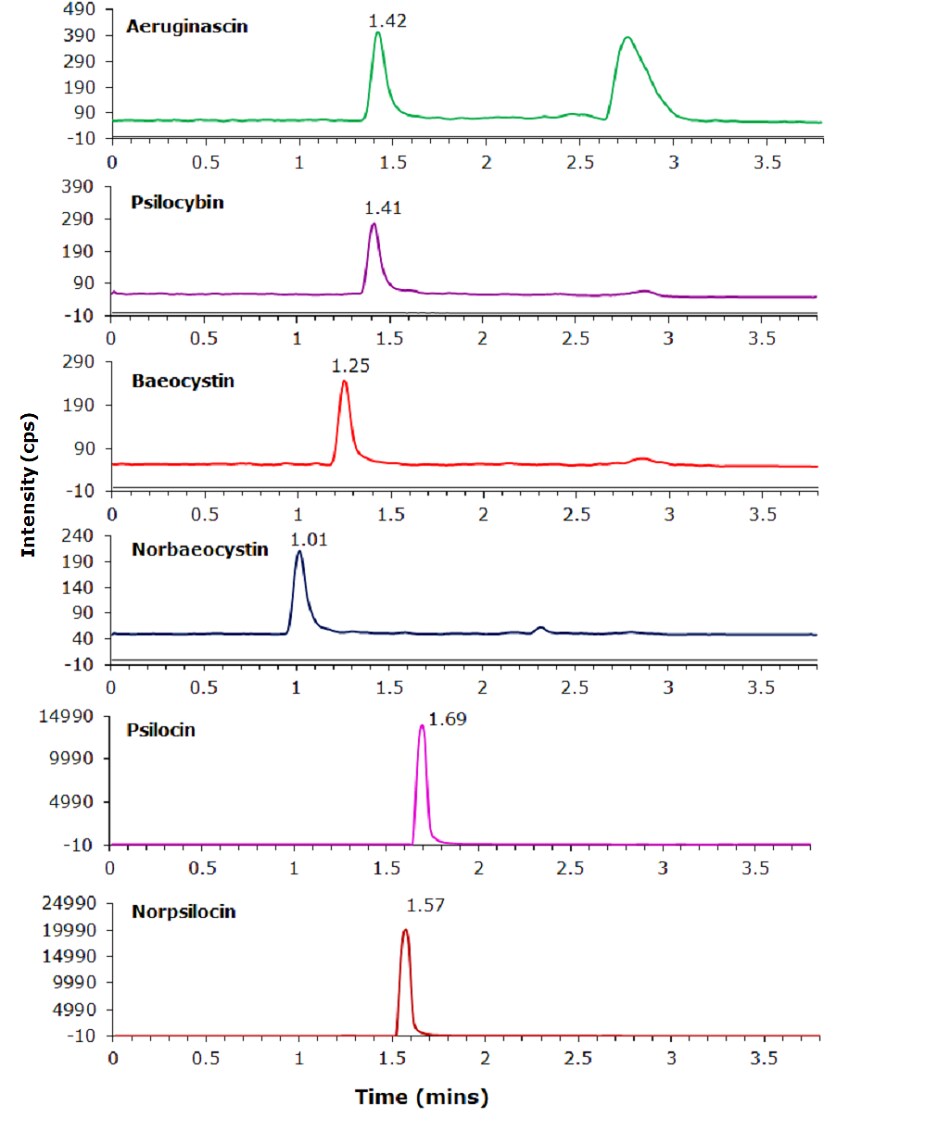
Figure 3.Psychedelic mushroom constituents chromatograms for monitored transitions.
Conclusion
Here, a method for the quantification of psilocybin and psilocin in ‘magic mushrooms’ was demonstrated. Lowering the upper limit to 25 ng/mL of the psilocin calibration curve and appropriately diluting the extracted sample is recommended to avoid signal suppression.
We show that psilocybin and psilocin are relatively stable up to room temperature conditions. Conversion of psilocybin to psilocin as well as degradation is observed in heated conditions at differing rates. Many factors can be the source of this variability, including air and photosensitivity that may have been introduced to the samples during packaging or the extraction process.4 We also illustrate the importance of preparing homogeneous batches to accurately determine the potency of a mushroom harvest. It can be concluded that within a single sample set, the sample extraction and analysis are reproducible.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?