Oligonucleotide Array CGH Analysis of a Robust Whole Genome Amplification Method
Introduction
In recent years, array-based Comparative Genomic Hybridization (aCGH) has been refined to determine chromosomal changes at progressively higher resolutions1. This evolving technology is, however, somewhat hampered by the large DNA input requirement—a minimum of 150,000 copies of a human genome, or 0.5 μg, are generally needed per sample to process one CGH array. GenomePlex® Whole Genome Amplification (WGA) provides a means to decrease the amount of required DNA for aCGH, which could expand the application of the technology to analysis of nanogram quantities of DNA, or even single cells. Furthermore, and perhaps more interestingly, GenomePlex WGA enables researchers to representatively amplify the genome from samples that have been fragmented to an average size of less than 1 kb. This feature may enable CGH analysis of formalin-fixed, paraffin-embedded (FFPE) samples that were previously thought to be unusable due to their high level of DNA degradation. In this study, we demonstrate successful use of WGA DNA on Agilent aCGH microarrays. The WGA product has also been demonstrated to work on BAC arrays 2, 3 and other oligo array4 platforms.
Materials and Methods
DNA Sources
Normal pooled genomic DNAs used in generation of Agilent data were purchased from Promega (product numbers G1471 (male) and G1521 (female)). HT29 (human colon carcinoma cell line) DNA used by Agilent was purchased from ATCC (product number HTB-38D).
Cell Culture and DNA Isolation
Human colon carcinoma HT29 cells (ATCC product number HTB-38) were cultured using McCoy’s 5a medium supplemented with 1.5 mM L-glutamine adjusted to contain 2.2 g/L sodium bicarbonate, 90%; fetal bovine serum, 10%. The genomic DNA was extracted using the GenElute™ Mammalian Genomic DNA Miniprep Kit (Product G1N70) and was used in MilliporeSigma’s samples. Genomic DNA from normal male was obtained from the whole blood of an individual without any history of cancer. The genomic DNA was extracted using the same genomic DNA isolation kit as described above and used in MilliporeSigma's samples.
Genomic DNA Fragmentation
Male and female genomic DNAs were intermittently sonicated using a sonicator probe (Digital Sonifier 450, Branson Ultrasonics Corp., CA) for 5 sec, 30 sec, 90 sec, or 120 sec. Fragmented DNAs were then run on a 1.2% agarose gel (E-gel Agarose Gels PN# G5000, Invitrogen, CA) to determine the fragment size distributions.
GenomePlex WGA
DNA samples were amplified using the GenomePlex WGA kit (Product WGA2). The amount of DNA input into WGA reactions was 50 ng, except for the titration experiment where the input amount was as indicated in the text below. After WGA, excess primers and dNTPs were removed by purifying the WGA product using the GenElute PCR Clean-Up Kit (Product NA1020).
DNA Labeling and Hybridization
Agilent’s Genomic DNA Labeling Kit PLUS (Agilent part number 5188-5309) was used to label the WGA DNA with either cyanine 3 or cyanine 5. As recommended by Agilent, 2.0 - 2.5 μg of amplified DNA was used as the input starting material for each labeling reaction. Alternatively, in the case of direct labeling (unamplified material), 0.5 μg of genomic DNA was used as the input starting material for the labeling reaction. Cyanine 3 and cyanine 5 labeled samples were mixed and hybridized to Agilent microarrays from either the Human Genome CGH Microarray Kit 105A (Agilent P/N G4412A) or the Human Genome CGH Microarray Kit 44B (Agilent P/N G4410B) following Agilent’s standard processing recommendations.
Microarray scanning and data analysis
Scanning and image analysis were conducted according to Agilent’s Oligonucleotide Array-based CGH for Genomic DNA analysis Protocol (version 4.0). Microarrays were scanned using an Agilent DNA Microarray Scanner (Agilent P/N G2565BA). Agilent’s Feature Extraction software (v9.1.3) was used to extract data from raw microarray image files in preparation for analysis. Agilent CGH Analytics software (v3.4) was used to visualize, detect and analyse aberration patterns from CGH microarray profiles.
Results
I. Input DNA Titration
Limiting amounts of starting template can inhibit array CGH analyses of certain precious samples such as laser capture microdissected cells or needle biopsies. The experiment described here was designed to demonstrate that GenomePlex WGA can be used to generate high-quality aCGH data across a range of genomic DNA inputs (1-100 ng). WGA yields for all inputs were in the range of 5-10 μg as measured by UV spectrophotometry (data not shown). DNA size distributions were between 200 and 5000 bp as measured by agarose gel analysis (Figure 1, lanes 4-7), although the size distribution for WGA product from reactions with 1 ng input was shifted slightly lower (Figure 1, lane 7). Microarray performance metrics values such as the Derivative LogRatio Standard Deviation (DLRSD), Signal-To-Noise Ratio (SNR) and Background Noise (BGNoise) demonstrated that the aCGH results from varying inputs into WGA reactions (1–100 ng input) were comparable (Table 1). The DLRSD value for the 1 ng input was higher than that for other inputs, but was within the acceptable range (see Table 1 footnote). The low probe-to-probe log ratio noise values indicate that chromosomal aberrations can be reliably detected across this wide range of input DNA masses.
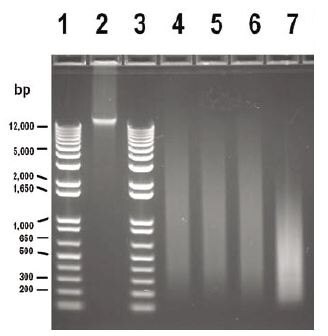
Figure 1. Gel Image, WGA Input Titrations. Equivalent amounts (1 μg/well) of amplified (200–5,000 bp) and unamplified (>12,000 bp) HT29 genomic DNA were compared via gel electrophoresis. Lanes 1 & 3: Molecular Weight Marker; Lane 2: HT29 DNA unamplified; Lane 4: HT29 DNA amplified from 100 ng input; Lane 5: HT29 DNA amplified from 50 ng input; Lane 6: HT29 DNA amplified from 10 ng input; Lane 7: HT29 DNA amplified from 1 ng input.
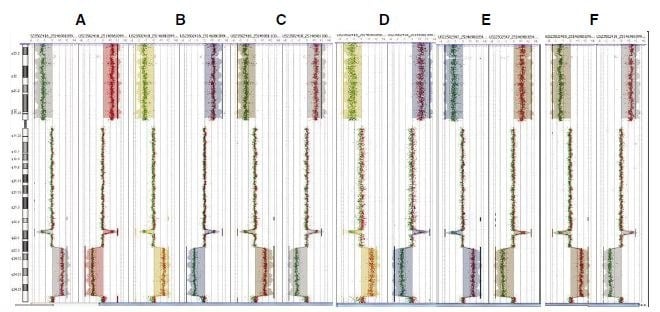
Figure 2.CGH Analytics ideograms illustrating input titration data from Agilent Human Genome CGH 105A microarrays. Dye swap plots were presented side-by-side in each panel (A-F). Polarity +1 (Cy5-HT29/Cy3-Female) is shown on the left and polarity -1 (Cy5-Female/Cy3-HT29) is shown on the right. These CGH plots compare normal female DNA vs. HT29 human colon carcinoma cell line having a known deletion across the p arm (horizontal shift to left of zero line), an amplification of the q arm in the q23.3-24.33 region (horizontal shift to right of zero line), and a focal deletion in q23.1. Dye swaps (switching the dye labels between samples) were performed on these samples to demonstrate that the results were not an effect of dye bias. A: amplified from 100 ng input, B: amplified from 50 ng input, C: amplified from 10 ng input, D: amplified from 1 ng input, E: MilliporeSigma amplified from 10 ng input, F: unamplified
*See Table 1 footnote for further explanation.
CGH Analytics software was used to analyze aberration patterns in HT29 DNA amplified across the range of inputs (Figure 2). Chromosome views for all input levels consistently revealed all known aberrations including a deletion across the 8p arm, an amplification of the 8q arm, and a focal deletion in 8q23.1. These data suggest that amplification integrity is maintained across a wide range of inputs into WGA. Array results generated by MilliporeSigma compare HT29 DNA to that from a single male patient without a history of cancer, whereas the Agilent data was generated by comparing chromosomal differences between HT29 and a pooled reference of healthy individuals. The MilliporeSigma data showed higher background signal values as well as lower signal-to-noise values for all inputs in comparison to the Agilent data (Table 1), which may be best explained by copy number variation; the pooled reference sample used in the generation of Agilent data eliminates much of this variation. In summary, the data indicates that when limited by the amount of available template, GenomePlex amplification from as little as 1 ng of starting genomic DNA can generate consistent CGH results.
II. Amplification from Fragmented DNA
Many researchers would like to perform aCGH on samples of questionable integrity, such as genomic DNAs from FFPE, frozen, and other archived tissues. The purposes of the following experiment were firstly to evaluate the feasibility of amplifying low molecular weight DNA using GenomePlex, and secondly, to determine if DNA amplified from fragmented DNAs would yield acceptable aCGH results. Sonicated DNA of varying sizes was used as a source for fragmented DNA. Size integrity distributions were determined by agarose gel analysis (Figure 3). WGA was performed using both intact and fragmented HT29 DNA and aCGH results are shown in Figure 4, revealing all well known HT29 aberrations on chromosome 8. Furthermore, the microarray data quality metric values were highly correlated between intact DNA and fragmented DNA (Table 2). The effects of fragmentation were further evaluated and verified by comparing male and female samples as recorded in table 2. These results indicate that sheared DNA of at least 400 bp in average size can be amplified and subsequently analyzed via Agilent microarrays to produce results comparable to those obtained using high molecular weight intact genomic DNA.
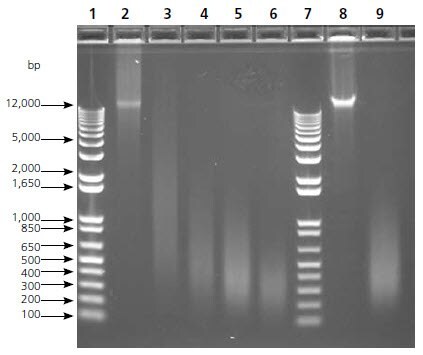
Figure 3. Gel Image, Sonication Time Course. DNA fragment size was determined via gel electrophoresis prior to amplification with GenomePlex WGA. Both human female genomic DNA and HT29 genomic DNA yield high molecular weight intact band (>12,000 bp), whereas the DNA sonicated intermittently for 5-120 sec yield multiple smaller DNA bands with varying sizes. The 90 sec sonication samples (~400 bp) were used in subsequent aCGH analysis. Lanes 1 & 7: Molecular Weight Marker; Lane 2: Intact Female DNA (>12,000 bp); Lane 3: Female DNA sonicated 5 sec (~1,500 bp); Lane 4: Female DNA sonicated 30 sec (~600 bp); Lane 5: Female DNA sonicated 90 sec (~400 bp); Lane 6: Female DNA sonicated 120 sec (~300 bp); Lane 8: Intact HT29 DNA (>12,000 bp), Lane 9: HT29 DNA sonicated 90 sec (~400 bp).
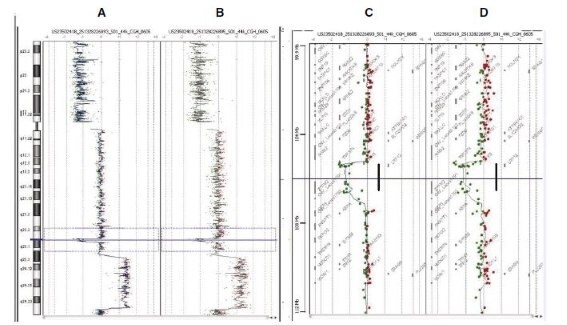
Figure 4. CGH Analytics ideograms illustrating data from Agilent Human Genome CGH 44B microarrays. WGA product generated from intact HT29 DNA (A) and fragmented HT29 DNA (B) revealed identical known deletion across the 8p arm (horizontal shift to left of zero line), amplification along the 8q arm in the 8q23.3-24.23 region (horizontal shift to right of zero line), and a focal deletion in 8q23.1 (horizontal shift to left of zero line, outlined by dotted blue box). Corresponding zoomed-in gene views for data from both intact DNA (C) and fragmented DNA (D) focusing on Chr8 q22.2-23.1; distinctly show a ~1.5 MB deletion (marked as thick line) involving the zinc finger protein ZFPM2 and tumor suppressor LRP12 that have been previously reported in BAC array-based analyses. A: Chromosome 8 view in HT29/Female with WGA of intact HT29 DNA (50 ng input); B: Chromosome 8 view in HT29/Female with WGA of fragmented HT29 DNA (50 ng input); C: Zoomed-in gene view of panel A (12 MB zoom window); D: Zoomed-in gene view of panel B (12 MB zoom window).
1Applies to male versus female comparison only. NA, not applicable.
*See Table 1 footnote for further explanation.
Conclusions
WGA product can easily be applied to Agilent’s two-color CGH array platform to yield high quality data. This amplification method allows for lesser amounts (nanogram quantities) of starting material. Furthermore, GenomePlex WGA represents an opportunity to amplify and analyze samples that have been severely fragmented. WGA DNA can be directly incorporated into the oligo-aCGH workflow without any deviation from Agilent’s recommended protocol. Amplified material produces high-quality chromosomal karyotypes that match results from unamplified genomic DNA samples. Future experiments will focus on producing high quality genomic profiles from tissue samples, including FFPE samples.
1 For male versus female comparison only. Not included in CGH Analytics 3.4 software QC Metrics.
Materials
References
GenElute is a a trademark of Merck KGaA, Darmstadt, Germany and/or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.
如要继续阅读,请登录或创建帐户。
暂无帐户?