Avanti Research™ FAQs
What are the Advantages/Disadvantages of Including Cholesterol in my Liposomes?
Cholesterol is a membrane constituent widely found in biological systems which serves a unique purpose of modulating membrane fluidity, elasticity, and permeability. It literally fills in the gaps created by imperfect packing of other lipid species when proteins are embedded in the membrane. Cholesterol serves much the same purpose in model membranes. Unfortunately, cholesterol presents certain problems when used in human pharmaceuticals. High purity sources suitable for clinical applications are not widely available. Most cholesterol commercially available is derived from egg or wool grease (sheep derived). These animal sources are potentially not suitable for human pharmaceuticals due to the potential viral contamination. Also, cholesterol is readily oxidized creating a stability problem for lipid based drug products. Some of these oxidation by-products tend to be rather toxic in biological systems. The oxidation products 25-hydroxy cholesterol, 7-keto-cholesterol, 7α- and 7ß-hydroxycholesterol, cholestane-3ß,5α,6ß-triol and the 5- and 7-hydroperoxides, were found in a concentrate which had activity causing aortic smooth muscle cells to die. This suggests that results from studies on atherosclerosis involving feeding experimental animals a diet containing cholesterol stored under adverse conditions (room temperature, open to air) could be ambiguous due to the potential presence of significant quantities of oxidized sterols.
How Do I Get My Hydrophobic Drug in The Lipid Membrane?
Hydrophobic drugs should be co-dissolved with the lipid(s) in an organic solvent to produce a homogeneous mixture. The organic solvent is removed and the lipid/drug residue processed as a typical liposome preparation.
What Issues Should I Consider When Selecting Lipids?
A. Phase Transition Temperature
The phase transition temperature is defined as the temperature required to induce a change in the lipid physical state from the ordered gel phase, where the hydrocarbon chains are fully extended and closely packed, to the disordered liquid crystalline phase, where the hydrocarbon chains are randomly oriented and fluid.1 There are several factors that directly affect the phase transition temperature including hydrocarbon length, unsaturation, charge, and headgroup species. As the hydrocarbon length is increased, van der Waals interactions become stronger requiring more energy to disrupt the ordered packing, thus the phase transition temperature increases. Likewise, introducing a double bond into the acyl group puts a kink in the chain which requires much lower temperatures to induce an ordered packing arrangement.
When developing a new product, procedure, or method, controlling the transition temperature of the lipid could be useful. Choosing a high transition lipid where the lipid vesicle would always be in the gel phase would provide a non-leaky packaging system. Alternatively, a lipid with a transition temperature between the starting temperature and the ending temperature of the system would provide a means of releasing packaged material as the lipid passes through its phase transition temperature and the vesicle becomes leaky. Also, one should consider how the transition temperature of the lipid could impact the processing steps. Using a high transition lipid when filtration is necessary could present some technical problems.
B. Stability
The long term stability or shelf-life of a drug product containing lipids can be dramatically affected by the lipid species used in the formulation. Generally, the more unsaturated a compound, the easier the product is oxidized, and thus the shorter the shelf life of the product. Lipids from biological sources (e.g., egg, bovine, or soybean) typically contain significant levels of polyunsaturated fatty acids and therefore are inherently less stable than their synthetic counterparts. While saturated lipids offer the greatest stability in terms of oxidation, they also have much higher transition temperatures and thus present other difficulties in formulation. If unsaturation is a requirement, keep the degree of unsaturation as low as possible. In most cases, compounds containing oleic acid (18:1, cisD9) are sufficient to satisfy the need for unsaturation, and since they are monounsaturated, oleoyl-containing products are much more stable than polyunsaturated compounds.
Stability issues due to hydrolytic degradation is a general problem with lipid products. Aqueous formulations of drug products tend to be less stable since the presence of excess or bulk water leads to rapid hydrolytic degradation in lipid preparations.3,4,5This hydrolysis is dependant on several factors including pH,3 temperature,3,5 buffer species,5 ionic strength, acyl chain length and headgroup,4 and the state of aggregation.4 A summary of the discussion on these factors can be found elsewhere.6 Others have shown that the cause of this hydrolysis is possibly due to the penetration of water into the membrane. Simon and McIntosh7report water penetration depths in PE and PE:cholesterol membranes determined by X-ray diffraction and specific capacitance measurements. In PE membranes, water penetrates to near the deeper carbonyl group, while in PE membranes containing cholesterol, water only penetrates to the glycerol backbone. This indicates that cholesterol could play a role in stabilizing lipid membranes to hydrolysis.
Stabilizing membranes has been the subject of research for many years. The bulk of this research has been aimed at stabilizing intact liposomes in the dry powder form such that they retain their trapped internal contents upon reconstitution. Recently, lipid preparations have been stabilized using carbohydrates.8,9 The possible reason for the stabilizing effect carbohydrates have on lipid membranes is that the carbobohydrate could intercalate into the headgroup region near the membrane/water interface and displace water from that region. In dry lipid formulations, this would serve to maintain a “hydrated” lipid membrane and keep the liposome structure intact. If this is true, it stands to reason that in an aqueous environment, the carbohydrate could still enter this region and displace water. This would tend to stabilize the membrane to hydrolysis from the bulk water phase.
C. Charge
Many biological membranes carry a net negative charge on their surface. The charge is generally imparted by the presence of anionic phospholipid species in the membrane. The major naturally occurring anionic phospholipids are phosphatidylserine, phosphatidylinositol, phosphatidic acid, and cardiolipin. Some bacterial systems also contain phosphatidylglycerol. The charge may provide a special function for the membrane. Several steps of the blood coagulation cascade require a lipid membrane. The assembling of protein aggregates on the surface of platelets requires a negatively charged surface. For the conversion of prothrombin to thrombin, not only does it require a negative surface, the requirement is somewhat specific, limited to phosphatidylserine (PS) and phosphatidic acid (PA).10 Coagulation proteins bind as tightly to negatively charged surfaces containing phosphatidylglycerol and phosphatidylinositol as they do to PS or PA membranes, however, the activity is only a fraction of that obtained with PS or PA membrane. Therefore, in some systems, not only must the charge requirement be satisfied, the system specificity for a particular species must be satisfied.
D. Lipid Mixtures
In many cases, a single lipid species does not yield the exact physical properties needed for a particular system, or does not adequately mimic the natural system for which it is intended to replace or reproduce. For these issues, consider a complex lipid mixture composed of two or more individual lipid species, the composition designed to create or reproduce a particular charge ratio, unsaturation ratio, phase transition temperature, or biological function. To reproduce the function of native brain tissue extracts, a blend of synthetic lipids (dioleoyl acyl composition) in the ratio 5:3:2 (wt%), PE:PS:PC, has been found to be satisfactory.11 This represents the general phospholipid composition of most brain tissues. Also, many commercially available coagulation reagents which contained crude brain extracts in the past are being replaced by synthetic lipid blends. The advantages to this replacement system are the increased stability due to the lack of polyunsaturated fatty acids found in biological extracts, and the reproducibility of synthetic blends. Blending of multiple lipid species does not require much additional effort in sample preparation. If the quantity of lipid blend is sufficient, many times the lipid supplier will pre-blend to the users specifications and provide a ready-to-use product.
E. Cholesterol
Cholesterol is a membrane constituent widely found in biological systems which serves a unique purpose of modulating membrane fluidity, elasticity, and permeability. It literally fills in the gaps created by imperfect packing of other lipid species when proteins are embedded in the membrane. Cholesterol serves much the same purpose in model membranes. Unfortunately, cholesterol presents certain problems when used in human pharmaceuticals. High purity sources suitable for clinical applications are not widely available. Most cholesterol commercially available is derived from egg or wool grease (sheep derived). These animal sources are potentially not suitable for human pharmaceuticals due to the potential viral contamination. Also, cholesterol is readily oxidized creating a stability problem for lipid based drug products.12 Some of these oxidation by-products tend to be rather toxic in biological systems. The oxidation products 25-hydroxy cholesterol, 7-keto-cholesterol, 7a- and 7b-hydroxycholesterol, cholestane-3b,5a,6b-triol and the 5- and 7-hydroperoxides, were found in a concentrate which had activity causing aortic smooth muscle cells to die.13 This suggests that results from studies on atherosclerosis involving feeding experimental animals a diet containing cholesterol stored under adverse conditions (room temperature, open to air) could be ambiguous due to the potential presence of significant quantities of oxidized sterols.
F. Source
There are two basic sources of phospholipids: synthetic and tissue-derived. Tissue-derived lipids are generally either egg-derived or bovine-derived. For clinical applications, either of these sources is not suitable due to stability problems and the possibility of viral or protein contamination. The U.S. Food and Drug Administration issued a letter restricting the source of bovine tissue used to isolate pharmaceutical products to countries and animals certified to be free of bovine spongiform encephalopathy (BSE). Cattle in the U.S. are not certified BSE-free and cannot be used to isolate pharmaceutical products. Egg sources are not currently restricted, however, additional testing for viral contamination may be required for pharmaceutical products. Regardless of the regulatory issues, animal-derived products do not offer any advantage to synthetic lipids. They are inherently less stable due to the polyunsaturated fatty acids, and in most cases the synthetic counterpart cost the same or less than the tissue-derived product.
Synthetic lipids from different sources are not necessarily equal either. Synthetic lipids can be prepared from glycerol or glycero-3-phosphocholine (GPC) derive from a plant or animal source. The latter is sometimes referred to as semi-synthetic lipids because a portion of the molecule is derived from a natural source. Lipids derived from glycerol require the chiral center be synthetically prepared which may lead to stereochemical impurities present in the final product. Lipids prepared using GPC obtained from an animal source may suffer from the same viral and protein contamination issues outlined above. The typical plant source for GPC is soybean lecithin.
Can I freeze my Liposomes?
In general, liposomes suspensions should not be frozen as the freezing process could fracture or rupture the vesicles leading to a change in size distribution and loss of internal contents. Depending on the application for the liposomes, changes in particle size can have a dramatic effect on functionality.
How Long Can I Store Liposomes?
Storage time depends on a number of factors including temperature, pH, medium, etc. Liposomes stored in a buffer at pH 7.4 and at ~4 °C did not display membrane structural changes for 5-7 days as demonstrated by retention of a trapped fluorescent marker. Beyond that time the fluorescent marker began to leak out of the liposome indicating the presence of membrane destabilizing components, presumably lyso lipid and free fatty acid generated by hydrolysis of the lipid.
What are the differences between Liposomes and Micelles?
Liposomes are composed of a lipid bilayer separating an aqueous internal compartment from the bulk aqueous phase. Micelles are closed lipid monolayers with a fatty acid core and polar surface, or polar core with fatty acids on the surface (inverted micelle).
How do I determine the size of my Liposomes?
There are several techniques suitable for determining the size of liposome preparations. The most straight forward is analysis by quasi-elastic or dynamic light scattering. This provides the mean diameter and distribution of the particles. It can also distinguish whether the particle population is uniformly distributed around one or more particle sizes (unimodal vs. bimodal). Particle size can also be determined by electron microscopy. This technique does not allow for good characterization of the distribution of particle sizes and may suffer from changes to particle size induced by sample fixation and staining. Finally, size can be determined by measuring the volume of trapped internal contents using a fluorescent probe. In this technique a fluorophore is trapped in the internal compartment of the liposome and the liposome separated from non-trapped fluorophore. The trapped fluorphore is released and concentration measured. This information is used to calculate the internal volume of the liposome and thus the size particle that matches that volume. This technique assumes the liposome is unilamellar and therefore is not suitable for multilamellar liposomes.
What is an SUV and LUV and how do they differ?
SUV are “small, unilamellar vesicles” or “Sonicated, Unilamellar Vesicles” and are usually prepared by sonication using a cuphorn, bath, or probe tip sonicator. LUV are “Large, Unilamellar Vesicles” and can be prepared by a variety of methods including extrusion (LUVET or “Large, Unilamellar Vesicles prepared by Extrusion Technique”), detergent dialysis (DOV or “Di-Octylglucoside Vesicles), fusion of SUV (FUV or “Fused, Unilamellar Vesicles”), reverse evaporation (REV or “Reverse Evaporation Vesicles), and ethanol injection. Unilamellar vesicles are prepared from MLV or LMV (Large, Multilamellar Vesicles), the large “onion-like” structures formed when amphiphilic lipids are hydrated. SUV are typically 15-30nm in diameter while LUV range from 100-200nm or larger. LUV are stable on storage, however, SUV will spontaneously fuse when they drop below the phase transition temperature of the lipid forming the vesicle.
Why doesn’t my SUV prep clear when I Sonicate?
There are many reasons for experiencing difficulty when sonicating lipid suspensions. The following list are some of the more common problems that may occur:
- In general, PC is more difficult to sonicate than charged lipids.
- Lyophilized powders disperse more readily and sonicate easier than lipid films dried from organic solvent.
- PE membranes will aggregate to form a flocculant.
- Charged membranes (PS, PG, PI, PA, etc.) will aggregate in the presence of divalent cations (e.g., calcium).
How do I Concentrate a Liposome?
There is not a good general procedure for concentration of liposomes. Large liposomal particles can be concentrated using centrifugation. The lowest speed possible to achieve pelleting is best since higher speeds could induce deformation and/or fusion of particles. Centrifugation is only possible with larger particles (>100nm). Small, sonicated (SUV) particles will not readily pellet due to their small size. Other methods of concentration may be possible but could lead to particle shape deformation depending on the sample and technique.
When I initially prepare my Lipids to form Liposomes, do I have to place the Lipids in a Vacuum to remove the Residual Chloroform?
It is necessary to put your lipid in a vacuum because residual chloroform can seriously alter the physical properties of the reconstituted membrane. To ensure consistent experimental results, it is necessary to remove chloroform and other organic solvents to a minimum amount such that it does not affect membrane properties. Blowing a stream of inert gas, i.e., nitrogen or argon, over your lipid solution simply removes the bulk organic solvent. Organic solvents can be trapped in the lipid, especially when the lipid dries as a film or oil. To remove the trapped solvent, it is necessary to subject your sample to a good vacuum. Avanti Research™ recommends at least 2-4 hours under vacuum for small samples (~ 1 mL) to ensure proper removal of solvents. Larger samples may require extended drying, i.e., overnight. Heating is usually not required provided your vacuum is sufficient (< 1000 mTorr). To minimize oxidation of unsaturated bonds, it is recommended the vacuum be purged with an inert gas.
I tried to weigh some Lipid Powder and it turned Gummy and Stuck to the Paper and Spatula. Is there a problem with the Lipid?
This behavior is typical of hygroscopic lipids. The hygroscopic lipids are generally those with short fatty acid chains (£C10) or double bonds in the fatty acid chain. This would include the short chain synthetic glycero-phospholipids (e.g., C6 PC, C8 PC, etc.), the unsaturated synthetic glycero-phospholipids (e.g., oleic, linoleic, arachidonic, etc.) and the non-hydrogenated natural glycero-phospholipids (e.g., Egg PC, Brain PS, etc.). These products should be weighed under dry conditions (i.e., in a dry box) or dissolved in an organic solvent and aliquoted as a solution (handling procedures for organic solutions of lipids apply).
Is it better to order my Lipids as a Powder or Chloroform Solution?
In general the powder form of the lipid is the most stable for storage, however, unsaturated lipids form hygroscopic powders which cannot be easily weighed. Therefore saturated lipids can be purchased as powders while unsaturated lipids should be purchased as the chloroform solution. If the lipid will be stored for an extended period of time, you may want to purchase the powder form for storage then dissolve the powder using chloroform when ready to use. To use the chloroform solution, aliquot a known amount of lipid (using stainless steel, glass, or teflon — DO NOT USE PLASTIC TUBES OR PIPET TIPS) into a glass vial and evaporate the chloroform using nitrogen or argon. The lipid film can be hydrated or resuspended in an appropriate solvent for lyophilization (see bulletin on Preparation of Liposomes).
What Is The Meaning Of “sn” In The Lipid Name?
From “Nomenclature of Lipids”, IUPAC-IUB Commission on Biochemical Nomenclature (CBN) (www.chem.qmul.ac.uk/iupac/lipid):
Lip-1.13. Stereospecific Numbering. In order to designate the configuration of glycerol derivatives, the carbon atoms of glycerol are numbered stereospecifically. The carbon atom that appears on top in that Fischer projection that shows a vertical carbon chain with the hydroxyl group at carbon-2 to the left is designated as C-1. To differentiate such numbering from conventional numbering conveying no steric information, the prefix ‘sn’ (for stereospecifically numbered) is used.
These Rules are as close as possible to the published version prepared by the Working Group on Lipid Nomenclature.
What do the numbers in the Lipid name mean?
The numbers in the lipid name are used to describe the fatty acid chains on the lipid. The numbers are generally presented in the format (number of carbons in fatty acid chain) : (number of double bonds in fatty acid chain), e.g., 16:0 would be 16 carbons in the fatty acid chain with zero double bonds, or the numeric representation of palmitic acid. Since glycerophospholipids contain two fatty acids per lipid, when only one number is given in the name it implies that both fatty acid are the same. The lipid 16:0 PC would be 16:0-16:0 PC, or dipalmitoyl PC. If there are two different fatty acids on the lipid, the name indicates both the fatty acid and the position. Therefore, 16:0-18:1 PC would represent 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine.
What Is the Correct Procedures for The Storage and Handling of Lipids?
Visit our Storage & Handling of Lipids page for more information.
How do I evaluate the Quality of my Lipid?
Periodic evaluation of product quality is recommended, and for most products the quality/purity of the lipids can be readily evaluated by TLC.
Can I Store my Lipid in an Aqueous Solution?
We do not recommend storing lipids in aqueous solutions. Note, the shelf life of lipids in aqueous solutions depends on a number of factors including temperature, pH, medium, etc. Lipids stored in a buffer at pH 7.4 at 4 °C are typically stable for 5-7 days. Beyond that time, the lipids slowly degrade generating the lyso lipid and free fatty acid via hydrolysis.
Can I store / handle my Lipid with Containers / Tools made of Plastic?
Lipids stored in water or buffered solutions are compatible with plastics, but organic solutions of lipids should never come in contact with polymer or plastic (polystyrene, polyethylene, polypropylene, etc.) as this will leach impurities although Teflon may be used. Extracted materials may affect the performance of the lipids.
How Can I Protect My Lipid From Oxidation?
Inert gases such as nitrogen or argon are used to protect compounds that are highly prone to oxidation. To store a material under inert gas, first choose a vial with a tight-fitting Teflon lid or cap and ensure the seal is as air-tight as possible. Aliquot your material as desired then direct a gentle stream of nitrogen or argon gas down into the vial for a few seconds, then cap the vial tightly and seal.
Do your Lipids contain BHT?
After purification, some products are stored as a chloroform solution containing BHT (to improve shelf-life). However prior to shipping, the BHT is removed by column chromatography; so any lipid Avanti Research™ ships does not contain BHT or any other antioxidant or preservative.
How do I Protonate an Acidic Lipid?
After purification, some products are stored as a chloroform solution containing BHT (to improve shelf life). However, prior to shipping, the BHT is removed by column chromatography, so any lipid Avanti Research™ ships does not contain BHT or any other antioxidant or preservative.
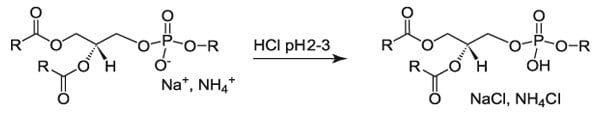
Theory
Protonate the phospholipid using acid. Wash the excess acid and salt from the product.
Equipment
- Separatory funnel (Teflon stopcock) or suitable glass vessel
- Rotary evaporator or nitrogen/argon stream
- pH indicating paper
Reagents
- Chloroform
- Methanol
- HCl
- Deionized water
Procedure
- Dissolve the lipid in a volume of chloroform: methanol (2:1 v/v) such that the lipid con centration is 10-50 mg/mL. (e.g. 9.0 ml)
- Add deionized water to the solution. (0.2 times the volume of the chloroform: methanol: e.g. 1.8 ml)
- Shake the solution and let the phases separate.
- Adjust the water phase to < pH 3.0 using acid. (pKa of the phosphate is approximately 3.5.)
- Shake the acidified solution and allow the phases to separate.
- Isolate the bottom phase. (This phase contains the acidified phospholipid.)
- Remove the chloroform by rotary evaporation or nitrogen/argon stream under a hood.
- Dissolve the residue in a suitable organic solvent.
Clarification on Packaging
To ensure we meet the minimum packaging requirements, we may over-pack our catalog products up to at least 10% (especially when dealing small unit sizes < 100 mg). Weights are determined by either physically weighting out (powders) or from measured aliquots from a gravimetrically determined solution and these are typically within 10%. For example, the concentration on the vial is correct (e.g. 5 mg/mL, 10 mg/mL, etc.), however, you will receive a volume greater than required (e.g. for 1 ml solution perhaps 1.1 to 1.3 mL).
Clarification on the Shelf Life
We guarantee the purity/stability of XXXXX for xxx months from receipt. The guarantee date for research compounds is the time Avanti Research™ guarantees the product to meet the release specifications when stored unopened at -20 °C ± 4 °C in its original container. The guarantee date for an Avanti Research™ product is not representative of the shelf life from the true manufacture date. The actual shelf life of the product may be well beyond the guarantee date. However, after the guarantee date, it is the customer’s responsibility to determine the quality of the lipids received, not Avanti Research™.
Clarification on Mycoplasma, Fungi, Endotoxin, etc.
Avanti Research™ does not test for mycoplasma, fungi, or endotoxin in any of our research products. Typically, we do not have issues with endotoxin contamination, but we cannot guarantee that our research products will be mycoplasma, fungi, or endotoxin free or sterile. However, Avanti Research™ can test for fungi or endotoxin levels and report on a CoA for any research products. If you are interested in this option, our QC group can provide pricing.
Clarification on Product Codes
The “C” stands for chloroform solution: the lipid is delivered dissolved in a chloroform solution.
The “O” designation on Avanti Research™ product codes refers to a product provided as a neat, solvent-free oil.
The “P” designation on Avanti Research™ product codes refers to a product provided as a neat, solvent-free, dry powder.
Clarification on the type of Chloroform used for Research Products.
We use B&J Brand® Chloroform, containing amylene and 1% ethanol preservatives, for all our research products, B&J chloroform is considered HPLC grade.
Clarification on Minor Fluorescent Contamination
We are aware of the minor fluorescent contaminant issue in many of our lipids. In response to this issue, we have developed a chromatography protocol that significantly reduces the fluorescent contaminants. However, there is an extra charge associated with the purification (approx. $300 per lipid, regardless of the amount of lipid ordered, if less than 10 g). For each item, there will be an additional item number of 999999, which will be associated with each product processing. Please call or email customer service to request this service when placing the order. Customer service can provide a ship date once the order is received.
Clarification on Container Packaging
Storage of organic solutions below -30 °C is not recommended unless the solution is packaged in a sealed glass ampoule. At low temperatures, Teflon cap liners may contract, causing leakage and/or exposure of the lipid to the atmosphere. As such, solution storage in the screw-top containers provided by Avanti Research™ are not recommended below -30 °C.
What is the Enantiomeric Purity of Avanti Research™ Phospholipids?
Avanti Research™ phospholipids are prepared using a precursor (GPC) derived from soybean lecithin, therefore the stereochemistry of the chiral carbon is dictated by the biological system from which it was derived. Since biological systems only manufacture the L-isomer, Avanti Research™ phospholipids are 100% L-isomer form. We have confirmed this by optical rotation and enzymatic digestion using phospholipase A2 (PLA2). PLA2 cleaves the sn-2 ester bond generating LPC and free fatty acid. The enzyme is specific for the L-isomer form, in fact the D-isomer acts as a competitive inhibitor. Treatment of Avanti Research™ synthetic PC’s results in complete digestion of the diacyl compound indicating absolute stereochemical purity. Spiking Avanti Research™ PC with 1% D-isomer PC as a control produces incomplete digestion (>1% residual diacyl PC remaining).
What is the Transition Temperature of the Lipid?
The phase transition temperature is defined as the temperature required to induce a change in the lipid physical state from the ordered gel phase, where the hydrocarbon chains are fully extended and closely packed, to the disordered liquid crystalline phase, where the hydrocarbon chains are randomly oriented and fluid. There are several factors that directly affect the phase transition temperature including hydrocarbon length, unsaturation, charge, and headgroup species. As the hydrocarbon length is increased, van der Waals interactions become stronger requiring more energy to disrupt the ordered packing, thus the phase transition temperature increases. Likewise, introducing a cis double bond into the acyl group puts a kink in the chain which requires much lower temperatures to induce an ordered packing arrangement.
A list of phase transition temperatures for selected lipids may be found on the following pages and sites:
- Phase Transition Temperatures for Glycerophospholipids
- Membrane Protein Data Bank (MPDB)
If the phase transition temperature is not listed on the above pages and sites then we must refer you to the literature. Note: Transition temperatures for natural products are typically not available in the literature. For processing (hydration and formulation) of natural lipids, we recommend the temperature of the medium should be above the gel-liquid crystal transition temperature (Tc or Tm) of any single lipid within the natural product.
What is the CMC of the Lipid?
The critical micelle concentration (CMC) of a surfactant is the concentration at which surfactant micelles form. Below this concentration, a surfactant exists as monomers in solution; at or above this concentration, surfactant micelles will be present. At concentrations below the CMC of a surfactant, surfactant molecules may bind to the hydrophobic portion of an integral membrane protein, making the protein soluble in aqueous solution, but the protein will not be dissolved in surfactant micelles. At surfactant concentrations above the CMC, integral membrane proteins will be dissolved in mixed micelles, containing protein and surfactant.
What is the pKa of the Lipid?
The pKa of the lipid is defined as the pH at which 50% of the lipids exists in the ionized form and 50% of the lipid exists in the nonionized form.
How do I Solubilize my Acidic Lipid (PG, PS, PA, etc.) in Chloroform?
Many long chain, saturated acidic lipids are difficult to solubilize in chloroform. We suggest adding a small amount of methanol (2%) and deionized or distilled water (0.5-1%).
I want to deliver my Lipid to Cells. What is the best Solvent to Dissolve the Lipid?
The solvents most used for delivery of lipids to biological systems are ethanol and dimethylsulfoxide (DMSO). Avanti Research™ does not use DMSO for any process; therefore, we do not have solubility data for our lipids using this solvent. Most of the lipids produced by Avanti Research™ are readily soluble in ethanol or mixtures of ethanol/water (up to 1:1, v/v). Some lipids may require heat and sonication to dissolve in ethanol/water.
My Lipid was in solution at room temperature but when I took the sample out of the freezer there was a precipitate in the vial. Is there something wrong with my lipid?
Some products manufactured by Avanti Research™ are readily soluble in organic solvents at room temperature but precipitate when stored at lower temperatures. The product should become soluble when removed from the freezer and warmed to room temperature. Some long-chain, saturated lipids may require heating the organic solution to 30-40 °C.
What is the Solubility of PIP2?
Avanti Research™ provides L-a-phosphatidylinositol-4,5-bisphosphate (#840046) dissolved in chloroform:methanol:water (20:9:1, v/v) which we find provides better solubility at higher lipid concentrations. If methanol and/or water present a problem in your system, it has been reported that an aliquot of the above solution can be evaporated (using nitrogen) and dried under high vacuum for 30 minutes, and dissolved in chloroform (3mL chloroform per mg lipid) (personal communication from Dr. Stuart McLaughlin).
How do I remove Solvent from a small amount of Lipid?
Avanti Research™ provides L-a-phosphatidylinositol-4,5-bisphosphate (#840046) dissolved in chloroform:methanol:water (20:9:1, v/v) which we find provides better solubility at higher lipid concentrations. If methanol and/or water present a problem in your system, it has been reported that an aliquot of the above solution can be evaporated (using nitrogen) and dried under high vacuum for 30 minutes, and dissolved in chloroform (3mL chloroform per mg lipid) (personal communication from Dr. Stuart McLaughlin).
My Lipid did not dissolve, what can I do?
Stock solutions of lipids in a biologically compatible solvent may precipitate when diluted with aqueous media. Precipitates often redissolve with sonication and/or warming. We recommend ensuring the precipitate has completely redissolved before using the solution (if possible). However, another option would be to deliver the lipid in suspension rather than as a true solution (sonicate the suspension immediately before dispensing to ensure a uniform suspension), or to deliver the lipid as a micelle or SUV.
How do I store my Lipid in an Organic Solution?
Phospholipids supplied as an organic solution should be stored in a glass container layered with argon or nitrogen at -20 °C ± 4 °C. Storage of organic solutions below -30 °C is not recommended unless the solution is packaged in a sealed glass ampoule. The closure for the vial should be lined with Teflon. Organic solutions should never be stored in polymer or plastic containers, or transferred with pipette tips (polystyrene, polyethylene, polypropylene, etc.) as this will leach impurities out of the plastics.
What is the purity of Avanti Research™ Lipid Maps Mass Spec Standards?
Generally, all qualitative and quantitative standards are >99% pure (unless specified). Mixed quantitative standards are available; for a full listing of Avanti Research™ pre-packaged Mass Spec standards, refer to our Mass Spectrometry Lipid Standards page.
What is the difference between Quantitative and Qualitative standards?
Qualitative standards are intended for the general identification of lipids by MS analysis. The pre-package qualitative standards are normally provided as powders and the amount varies according to the product. Note that qualitative standards are suitable for LC/MS development and optimization. We can provide a certificate of analysis on request for the qualitative standards but are not as extensive as those of the quantitative standards.
Quantitative standards are characterized and pre-packaged in unit containers at defined concentrations. A detailed Certificate of Analysis accompanies the standard, and the stability is monitored by our QC staff. The quantitative standards are pre-packed into single-use ampoules at known concentration in a solvent such as methanol or ethanol.
These qualitative and quantitative standards were specifically designed for Lipidomic applications.
Can I use my LIPID MAPS Mass Spectrometry Internal Lipid Standards more than once?
Avanti Research™ LIPID MAPS Mass Spectrometry Internal Lipid Standards are designed to be “one-time use items”. It is important to directly transfer from the ampoule to the experiment or prepare as a dilution for immediate use. Avanti Research™ cannot guarantee product purity and subsequent performance if used outside these guidelines.
Please note, if you need larger quantities of standards, all of our research catalog items are suitable for use as MS standards. Avanti Research™ can provide a Certificate of Analysis for all research products.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?