Protein- and Peptide- Polymer Conjugates by RAFT Polymerization
The modification of biomacromolecules, such as peptides and proteins, through the attachment of synthetic polymers has led to a new family of highly advanced biomaterials with enhanced properties. These materials were first developed to enhance the circulation time of proteins in vivo through protection with covalently attached poly(ethylene glycol) (PEG), a process now comonly know as PEGylation. The conjugation of biomolecules to polymers has received a surge of interest over the last few years, triggered by new and versatile polymerization techniques that enable beneficial polymer functionalities and advanced material properties.
Polymer conjugates of biomolecules can be produced using either the convergent approach, in which the polymer is synthesized separately prior to conjugation with the biomolecule, or by the divergent methodology, in which the polymerization reaction occurs in presence of the biomolecule (Figure 1).1 The latter approach can be further divided into a graftingfrom method, in which the biomolecule is functionalized with one or more units in order to grow a polymer chain, and a grafting-through method, in which the biomolecule is connected to a polymerizable unit that can be incorporated with other monomers to yield a bottle brush polymer decorated with biomolecules. Among the various polymerization techniques employed for the synthesis of these materials to date, reversible addition-fragmentation chain transfer (RAFT) polymerization has emerged as one of the most versatile and powerful processes. RAFT polymerization relies on a simple setup, versatile reaction conditions, and provides access to a myriad of functionalities, making it an ideal method for biomolecule modification.
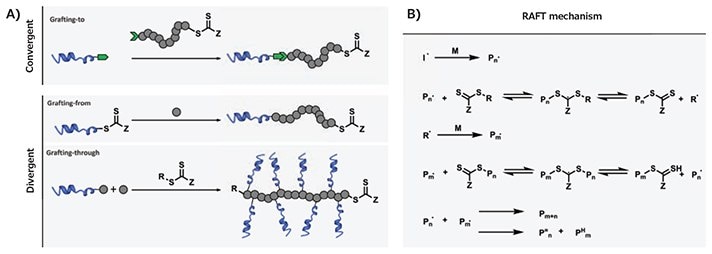
Figure 1. A)Possible strategies for the synthesis of peptide-polymer conjugates using a RAFT methodology. B) Mechanism of RAFT polymerization.
Divergent Strategies to Peptide-Polymer Conjugates
The divergent approach has several specific advantages over convergent approaches for the synthesis of highly-defined, biomolecule–polymer conjugates. For example, the attachment of a small initiator molecule to the biomolecule allows for greater control compared to the conjugation of a polymeric chain, leading to better overall control over the number of polymers attached. In addition, purification is simplified since the removal of the polymeric species is not necessary.2 However, the major disadvantage of the divergent approach is that the biomolecule must be compatible with the polymerization conditions, as it is involved in all synthetic steps.
RAFT polymerization is an ideal synthetic method for biomolecule-polymer conjugates because it is a robust and versatile process.3 Radical polymerization processes are usually compatible with peptides or proteins since the propagating radical is non-reactive with the majority of functional groups associated with these biomolecules. Furthermore, RAFT polymerization enables polymerization of a wide range of monomers and does not require the use of a catalyst, avoiding the need for conjugate purification after the polymerization reaction. RAFT does require an external radical source, such as a thermal radical initiator (I), but the amount required is usually negligible especially in the case of high Kp monomers (e.g., acrylamides) allowing for high chain transfer agent/initiator (CTA/I) ratios to be used.4
As the divergent methodology requires biomolecule compatibility with polymerization conditions, polymer synthesis is ideally carried out in aqueous solution. RAFT is compatible with an aqueous environment and a large variety of hydrophilic monomers can be polymerized using water-soluble chain transfer agents (CTAs).5 The reaction temperature must be kept low to reduce thermal degradation of the proteins or peptides during the polymerization process. This can be accomplished using a radical initiator with a low decomposition temperature (e.g., VA-044) or alternative radical sources, such as photoinitiators or suitable redox pairs.6
Another criteria to consider when using a divergent strategy in RAFT polymerization is CTA stability. Many RAFT agents (e.g., dithiobenzoates), are instable in the presence of nucleophiles, such as the primary amine of lysine, which are very commonly found in biomolecules. One strategy is the use of CTAs that are less sensitive to nucleophiles, such as trithiocarbonates. In addition, altering the solution pH during polymerization to ensure the protonation of a majority of amine functional groups, enables the growth of RAFT-based polymers without interference from the protein. Free thiols found in proteins, such as those present in cysteine, can also interfere with RAFT-based polymerization processes as thiols can react irreversibly with a propagating radical and lead to the termination of a growing chain. In the case of synthetic peptides, protecting groups can be used and removed post-polymerization.
RAFT-Based Grafting-From Methodology
To facilitate a grafting-from approach using RAFT polymerization, the CTA must be immobilized on the target peptide or protein, which can be performed via either the R- or Z-group (Figure 2).7 If the R-group is utilized, the biomolecule acts as the initiator and the polymeric chain remains attached to the biomolecule throughout the entire process. While this method yields more stable conjugates, termination of a propagating radical leads to dead chains associated with the biomolecule, in which polymerization cannot be reinitiated.
If the CTA is attached via its Z-group, the polymer will grow in solution and reattach to the biomolecule (which carries the CTA) only during the chain transfer event. In this approach, a high livingness of the polymer attached to the biomolecule is achieved,8 as dead chains will not reattach to the protein. However, especially in the case of long polymers, the reattachment step is sterically challenging, and can lead to a decrease in the number of polymer arms per biomolecule. In addition, the sensitivity of CTAs to nucleophiles renders Z-group conjugates comparably instable. While this instability may present issues in some applications, facile detachment of the polymer from the conjugate allows for ease of polymer analysis (e.g., size distribution).
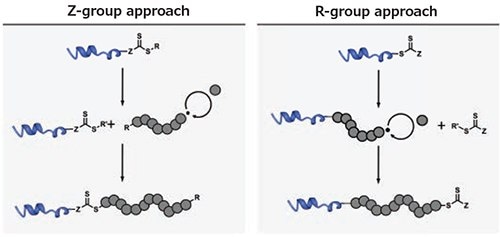
Figure 2.Comparison of the R- and Z-group approach using a RAFTbased grafting-from technique. The R’- leaving group of the chain transfer agent after the addition-fragmentation event has been omitted for clarity.
CTA molecules, such as dithiobenzoates or trithiocarbonates, are compatible with solid-phase peptide synthesis and can be attached to the N-terminus of a peptide on resin. After cleavage and polymerization, the peptide protecting groups are removed to yield the final conjugate.9,10 Alternatively, the CTA can be attached to the pendant groups of peptides (e.g., glutathione or cyclic peptide nanotubes) as sites for polymer chain propagation.11,12 RAFT-based, grafting-from approaches from proteins were demonstrated using BSA13 and lysozyme,14 utilizing either free thiol or amine groups and using either the R- or Z-group.15 The preparation of block copolymer-based conjugates using this approach has also been described.16
RAFT-Based Grafting-Through Methodology
The grafting-through approach requires functionalization of the peptide with a polymerizable group, such as an acrylate-based or styrene moiety, and is usually done as the last step of solidphase peptide synthesis. These macromonomers can then be polymerized to yield densely peptide-functionalized polymers. This method has been used to create stimuli-responsive peptide brushes by encorporating β-sheet forming sequences.17,18
Convergent Strategies to Peptide-Polymer Conjugates
A convergent, or grafting-to, approach is accomplished by reacting a specific protein site either to polymer pendant chains or to the RAFT agent at the chain end. Advantages of this method include the ability to use high temperature and organic solvents during the RAFT polymerization step, and easy characterization of the protein and polymer prior to coupling. In contrast, this approach requires high coupling efficiency and mild grafting conditions to retain protein activity. Due to the lack of diversity in naturally available functional groups on protein surfaces, reactions used in the graftingto step were initially limited to reactions with amine and thiol groups. However, the introduction of unnatural amino acids via bioengineering techniques or post-translational modification procedures has drastically increased the number of conjugation techniques available.
Conjugation Methods Using Amines
Primary amines (e.g., ε-amino group of lysine residues) are highly abundant on the surface of most proteins. The most common conjugation method for amine groups utilizes RAFT polymers modified with activated esters to form a stable and inert amide bond with the protein (Figure 3). Activated esters derived from N-hydroxysuccinimide (NHS, Prod. No. 130672) are often used due to mild coupling conditions and high coupling efficiency. For example, NHS derivatives of acrylic acid are easily copolymerized with other monomers via RAFT, offering a convenient handle for post-polymerization modification. Controlling the ratio of NHS-bearing monomers incorporated in the polymer chain can be used to control the degree of proteinpolymer functionalization.19 While NHS groups feature facile conjugation chemistry, they also have a tendency to hydrolyze in aqueous solutions, and replacement with more stable alternatives, such as pentafluorophenyl methacrylate (Prod. No. 741108) is often a good option.20 Alternatively, RAFT CTA containing activated esters can be used to graft a single protein at the end of the polymer chain. CTA containing α-carboxylic acids can easily be converted into active esters through a reaction with NHS, either pre- or post-polymerization.21 Protein conjugation can then be performed in slightly alkaline water or in DMSO with an organic base, such as N,N-diisopropylethylamine (Prod. No. 496219), to limit hydrolysis. Water-soluble, amide-promoting coupling agents (e.g., 1-ethyl-3- (3-dimethylaminopropyl)carbodiimide [EDC, Prod. No. 39391]) can also be used as an alternative to activated esters, directly reacting α-carboxylic acids from RAFT polymers to free amine groups on proteins.22 Additionally, particularly sensitive proteins, such as enzymes, can be coupled to RAFT polymers prepared using a CTA-bearing 2-mercaptothiazoline to create a biodegradable linker, allowing for the recovery of protein activity following linker cleavage.23
Conjugation Methods Using Cysteines
Cysteine residues are far less abundant than lysine on the surface of biomacromolecules, reducing the risk of interference with the biological activity after polymer conjugation. The simplest method of functionalization is the formation of disulfide bonds, typically using activated disulfide residues, such as pyridyl disulfide (Figure 3). Again, two major approaches can be utilized. Pyridyl disulfide-bearing monomers, such as pyridyl disulfide methacrylamide, can be copolymerized using RAFT to yield copolymers that can be decorated with multiple copies of thiol-bearing proteins.24 In contrast, a pyridyl disulfide-bearing CTA agent allows for the grafting of a single protein copy onto a polymer. For example, 3-(pyridin-2-yldisulfanyl) propyl 2-(ethylthiocarbonothioylthio) propanoate was used to conjugate polymers to a variety of substrates ranging from glutathione to large proteins, such as basic fibroblast growth factors.25 While disulfide bonds are useful for preparing bioresponsive systems, their sensitivity to reducing agents can lead to the premature cleavage of protein-polymer conjugates in some applications.
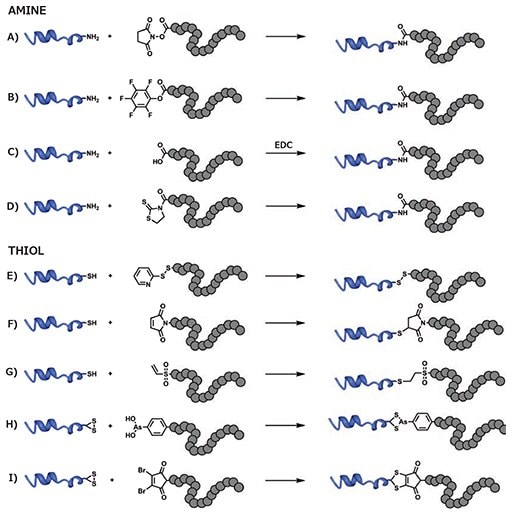
Figure 3.Conjugation of RAFT polymers to either lysine residues (amine) or cysteine residues (thiol) of proteins via A) reaction of an amine with an N-succinimidyl activated ester, B) reaction of an amine with a pentafluorophenyl activated ester, C) reaction of an amine with a carboxylic acid with EDC activation, D) reaction of an amine with a mercaptothiazoline ester, E) disulfide formation between a thiol and pyridyl disulfide, F) Michael addition of a thiol to a maleimide, G) Michael addition of a thiol to a vinyl sulfone, H) disulfide bridging using an arsenous acid, I) disulfide bridging using a dibromomaleimide.
A more stable alternative exploits the Michael addition of a thiol group to an α-β unsaturated carbonyl (e.g., maleimide or vinyl sulfone). RAFT polymers have been conjugated to bovine serum albumin (BSA) or ovalbumin using a 1,8-bismaleimidodiethyleneglycol linker.26 Another technique uses a CTA bearing a protected maleimide moiety for postpolymerization functionalization. For example, a furanprotected, maleimide-terminated analog of 4-cyanopentanoic acid dithiobenzoate (CPADB) was obtained in two steps from 10-dioxatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione and used to prepare maleimide-terminated poly(methyl methacrylate) (PMMA). This functionalized polymer was subsequently conjugated to albumin by simply stirring in PBS buffer.27 To avoid the protection and deprotection cycle, the α-β unsaturated carbonyl moiety can be added to the polymer after the polymerization step.28
Enhanced specificity can be obtained by targeting native disulfide bonds in proteins instead of isolated thiol residues. This has been successfully achieved using a trivalent derivative of arsenous acid and RAFT polymerization.29 The use of dibromomaleimide functional groups, however, has only been used in conjunction with atom transfer radical polymerization (ATRP) and remains to be implemented with a RAFT-made polymer.30
Conjugation Methods Using Unnatural Amino Acids
To achieve site-selective ligation, a range of bioorthogonal reactions have been developed that utilize unnatural amino acids (UAA). While incorporation of UAA in a peptide sequence can be easily achieved via solid-phase peptide synthesis, UAA incorporation in proteins requires either post-translational modification or genetically encoding UAA incorporation during bacterial expression.31
“Click” reactions, such as copper(I)-mediated azide-alkyne cycloaddition (CuAAc), are perfectly suited for bioconjugation as they feature orthogonal reactivity to biological functional groups, high conversion, limited side reactions, and compatibility with aqueous conditions. Using (prop-2-ynyl propanoate)yl butyltrithiocarbonate as the CTA, Perrier et al. prepared alkyneterminated RAFT polymers that were later coupled to a peptide bearing an azide functional group (Figure 4).32 The reverse can also be synthesized by ligating an azido-bearing polymer to a protein in which a cysteine was modified with propargyl maleimide.33 This reaction, however, has several disadvantages including the lack of full conversion without microwave irradiation and the use of a toxic copper catalyst, which could interfere with subsequent biological applications. A more biocompatible approach uses strained cycloalkynes or cycloalkenes that can react with azides or tetrazines, respectively, in the absence of copper catalyst. A maleimide-trans-cyclooctene linker was used to modify the cysteine residue of a lysozyme protein mutant, and later used to couple two copies of the protein to a tetrazinebearing polymer.34 Strained alkynes can also be attached to the CTA prior to RAFT polymerization. For example, poly-N-(2- hydroxypropyl)methacrylamide (PHPMA) polymerized using an α-dibenzocyclooctyne-containing CTA was directly conjugated to an azide-modified peptide, after polymerization.35
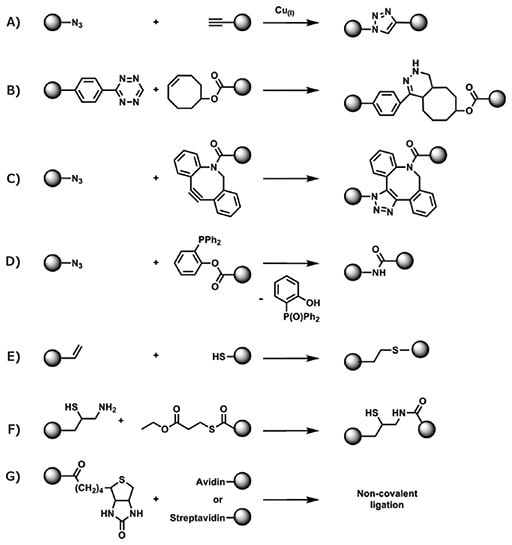
Figure 4.Conjugation of RAFT polymers to non-naturally occurring functional group of proteins via A) copper(I)-mediated azide-alkyne cycloaddition, B) metal-free tetrazine−trans-cyclooctene ligation, C) metal-free ligation of dibenzocyclooctyne to an azide, D) Staudinger ligation of an azide with a phosphine, E) thiol-ene ligation, F) native chemical ligation of a thioester to a terminal cysteine, G) non-covalent ligation based on affinity of biotin with avidin or streptavidin. The spheres in this scheme are interchangeable and can represent either the polymer or the protein molecules.
As a highly specific reaction, a Staudinger ligation is used to bind an azide selectively to a phosphine group. For RAFT applications, the phosphine can be introduced to the α-carboxylic acid of common CTAs via reaction with 2-(diphenylphosphanyl)phenol36 allowing for quantitative ligation to azide-containing macromolecules in an aqueous environment. For shorter peptides, thiol-ene click chemistry can be exploited to couple an allyl oxycarbonyl-protected lysine residue to a RAFT-synthesized polymer, which had undergone aminolysis.37 Native chemical ligation can also be used to conjugate a peptide thioester to a RAFT polymer modified to incorporate a single pseudo-cysteine monomer.38
Finally, an elegant way to conjugate polymers and proteins exploits the inherent affinity of biotin, a co-factor involved in multiple eukaryotic biological processes, to avidin or streptavidin proteins. Due to its relatively small size, biotin can easily be introduced onto a CTA prior to RAFT polymerization.39
Additional information about common grafting-to methods can be found in the a recent, comprehensive article by Vanparijs et al.40 New directions in protein-polymer conjugates synthesis have exploited orthogonal combinations of these reactions to generate more advanced materials by attaching different proteins to the same polymer. For example, Maynard et al. successfully prepared a poly-NIPAM polymer utilizing a dual functional RAFT chain transfer agent to yield a polymer with biotin on one end and a maleimide group on the other.41
Conclusion
The versatility of the RAFT process has made it an ideal tool for the modification of biomolecules, particularly peptides and proteins. RAFT not only enables the introduction of functional groups and novel properties to peptide and proteins, but also the versatility of the process permits a flexible synthetic approach for bioconjugates. With a large variety of established synthetic techniques now available, complex conjugate structures have created a path for a wealth of applications in a variety of fields.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?