氧化石墨烯和还原氧化石墨烯的应用
简介
氧化石墨烯(GO,产品编号:763705和777676)是一种独特的材料,可以看做连接有多种氧基官能团的单分子层石墨,官能团包括环氧基、碳基、羧基和羟基。1,2,3当一种单层石墨——石墨烯,被首次分离和研究后,GO受到的关注显著增加。4最初研究者希望GO可以成为石墨烯的合成前体。5 当绝缘的GO被还原后,得到的还原氧化石墨烯(GO,产品编号:777684)类似于石墨烯,但含有残余的氧和其他杂原子,并且具有结构上的缺陷。6,7合成出更接近原始的石墨烯的rGO,是本领域内的一项极具创意的挑战。虽然如此,由于rGO可以由GO的水分散溶液制成薄片,并具有适度的导电性,将其用于电子设备是极具吸引力的。8,9,10,11,12除了作为电子设备的组件之外,GO和rGO也被用于纳米复合材料13,14、聚合物复合材料13、能量存储14、 生物医学15,16,17、催化剂18,19,20 和表面活性剂21,这些领域之间存在一定程度重叠。
GO的合成和还原
目前大部分GO的合成工艺都基于Hummers最先发表的方法,即使用高锰酸钾硫酸溶液氧化石墨。22有报道称可以使用肼还原GO,5 但是,肼具有强毒性并可能使GO连接上氮基杂原子官能团。23因此,NaBH4、NaBH414、抗坏血酸24、24 HI25,26和其他物质可替代肼用于GO的还原。GO可以以薄片或者水溶液的形式被还原。还原的方法近期已有回顾。6
电子设备
已有多种电子设备使用GO作为其至少一种组件的原始材料。其中一种设备是石墨烯制成的场效应晶体管(GFET)。27,28采用rGO的场效应晶体管(FET)已经被用作化学传感器29,30,31和生物传感器。图1即为一种GFET传感器的示意图。31使用官能化rGO作为半导体的GFET 已被用作生物传感器,用于检测激素儿茶酚胺分子32、抗生物素蛋白33、和DNA34。在其他研究中,葡萄糖氧化酶官能化的GO被沉积在电极上用作电化学葡萄糖传感器。35
可见光透明电极对于发光二极管(LED)和太阳能电池设备都很重要。由于GO可被制成溶液,相对于其它透明电极例如ITO,在这些设备中使用rGO作为透明电极更加方便。36,37除了透明电极外,rGO还被用作聚合太阳能电池和LED的空穴传输层。38,39
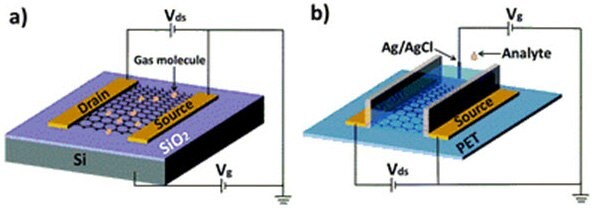
图 1.(a) Si/SiO2 衬底上的典型的背栅结构GFET,可用作气体传感器。(b) 柔性聚对苯二甲酸乙二醇酯(PET)衬底上的典型的液体栅结构GFET,可作为化学和生物传感器,用于水溶液中。经法国国家科学研究中心(CNRS)和英国皇家化学学会许可,转载自参考文献31。
能量存储
纳米复合rGO材料已经被用于锂电子电池中的大容量能量存储。研究发现,电绝缘的金纳米属氧化颗粒被吸附到rGO上后可以提高这些材料在电池中的性能。40,41,42,43,44例如,与纯的Fe3O4或Fe2O3相比,将Fe3O4吸附到rGO上可以提高其储能容量和循环稳定性(图2)。43使用微波法可以剥离和还原GO得到大表面积的rGO, 后者可用作超级电容器中的储能材料。45,46
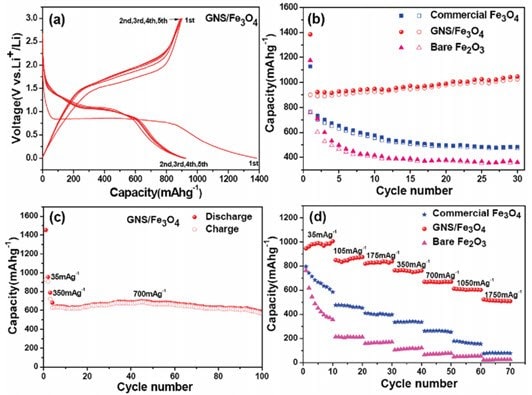
图 2.(a) GNS/Fe3O4复合物的放电/充电曲线。(b) 商业化Fe3O4颗粒、GNS/Fe3O4复合物和未改性Fe2O3颗粒在电流密度为35 mA/g下的循环性能曲线,实心符号表示放电,空心符号表示充电。(c) GNS/Fe3O4复合物在电流密度为700 mA/g下循环100次的循环性能曲线。(d) 商业化Fe3O4颗粒、GNS/Fe3O4复合物和未改性Fe2O3颗粒在不同的电流密度下的充放电速度性能曲线。GNS = rGO。授权转载自参考文献43。2010美国化学学会版权所有。
生物医学应用
GO在生物医学领域的一项应用是作为药物传输系统的一个组成部分。 官能化纳米氧化石墨烯(nGO,品编号:795534)已经用于抗癌药物靶向传输的多个研究中。SN38 — 一种喜树碱(产品编号:H0165)衍生物可吸附在聚乙二醇(PEG)官能化的nGO表面上,形成nGO‒PEG‒SN38,以提高药物在水和血清中的溶解性。47该研究显示,在降低人结肠癌细胞系HTC-116的细胞活力方面,相较于伊立替康(CPT-11)——一种FDA认证的SN38前体药物,nGO‒PEG‒SN38的有效性提高了三个数量级(图3)。47如图3所示,nGO‒PEG‒SN38的有效性与DMSO中的SN38相似。47PEG和透明质酸官能化的nGO经皮传输并配合近红外激光,可用于小鼠黑色素瘤皮肤癌的光热消融治疗。48在一项单独的研究中,磁铁矿被吸附到装载有抗癌药物盐酸阿霉素(DXR,产品编号:D1515和44583)的GO上,继而用磁铁使得药物可以被靶向运输到特定部位。49
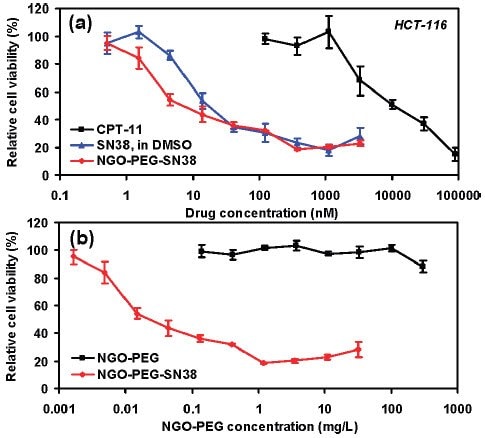
图 3.细胞体外毒性试验。(a) HCT-116细胞在不同浓度CPT-11、SN38和NGO‒PEG‒SN38中培养72小时的细胞相对活性曲线(相对于未处理对照品)。游离的SN38被溶解于DMSO中并用PBS稀释。水溶性的NGO-PEG-SN38显示出与SN38在DMSO中相似的毒性,并且效能远高于CPT-11。(b) HCT-116细胞在吸附(红色)或者未吸附(黑色)SN38的nGO-PEG中培养后的细胞相对活性数据。普通的NGO-PEG即使在很高浓度下也未显示有明显的毒性。误差线基于三次重复试验。NGO = nGO。授权转载自参考文献47。2008美国化学学会版权所有。
生物传感器
GO和rGO已用于若干检测生物相关分子的系统中。由于具有荧光共振能量转移(FRET)特性,GO已用作生物传感器中的荧光猝灭材料。在Lu等进行的一项研究中发现,具有荧光标记的单链DNA(ssDNA)与GO非共价结合,随后荧光标记淬灭。50 添加互补的ssDNA以移除GO表面被标记的DNA之后,荧光恢复。利用其FRET特性,还可以与荧光标记的ATP适配子联合,以检测出低至10 μM的ATP。51叶酸官能化的GO被用于检测人宫颈癌和人乳腺癌细胞。52
我们提供高品质氧化石墨烯产品以满足您对创新和先进材料的研究需求。有关我们的石墨烯和氧化石墨烯产品列表,请访问sigmaaldrich.com/nanocarbons。
产品列表
参考文献
如要继续阅读,请登录或创建帐户。
暂无帐户?