Aseptic Process Simulation in the Pharmaceutical Industry
Superior Quality Culture Media for Reliable Aseptic Process Simulation (APS)
When performing Aseptic Process Simulation (APS), also known as media fill test, you shouldn’t have to worry about culture media compromising your validated process. Have peace of mind with our irradiated granulated and ready-to-use liquid culture media. Every batch is carefully tested for sterility and growth performance. All our culture media are supplied for the ultimate security of your aseptic process.
Culture Media for Aseptic Process Simulations (APS) include the following features:
- Choice of compositions: Standard Tryptic Soy Broth (TSB) or non-animal peptone broth
- Flexible size and formats: Granulated culture media in 500 g or 5 kg triple-wrapped drums; ready-to-use liquid media in 10 L bags; and customized sizes upon request
- Validated sterilization process
- Free of viable Mycoplasma and BSE
- Triple-wrapped for safe transfer to cleanrooms
- Proven growth performance exceeding EP/USP standards

- Reduced dust spread during preparation lowers health risks and prevents environmental contamination. Less dust on equipment also eases handling and cleaning.
- Excellent solubility significantly reduces preparation time and effort.
- Optimized cold filtration properties, from raw materials to final product, help prolong filter life and reduce replacements, saving time and costs.
- Irradiated at a high dose to reduce microbial cross-contamination and risk of false positives due to irradiation survivals.
- Growth promotion and sterility performance in compliance with EP/USP and ISO® EN 11133 guidelines regarding production and performance testing.
Optimized Cold-filtration Avoids Clogs and Costs
Most aseptic filling processes involve a sterile filtration step, which is typically part of the process simulation with culture media. The large amounts of liquid running through the filters make this step both time-consuming and expensive. This is of particular concern when problems, such as clogs, occur. Download our Cold Filterability Study
To avoid such challenges, we take every measure to ensure the reliable cold-filtration properties of its granulated culture media. Filtration tests are performed at every stage and for every batch:
- Qualification of raw materials
- Control of incoming peptones
- Pre-batch samples and in-process testing
- End-product testing

Our ready-to-use culture media include Tryptic Soy Broth (TSB) prepared according to European and US pharmacopeias. Alternatively, you can also choose our Vegetable Peptone Broth (VPB). These liquid broths are first sterilized by autoclave, and then undergo a two-step pre-filtration process consisting of a particulate filter to avoid clogging, and a 0.2 μm filter for sterilization. The media is supplied in 10-liter bags that are triple-layered and irradiated for enhanced safety.
Gas-impermeable Bags for Greater Safety and Convenience
The self-collapsing bags include an 80 cm long tube with an MPC connector (male insert 3/8”) or a sterile Lynx® connector (for Vegetable peptone broth only), which enables direct connection to the filling line. Various types of connectors are available upon request. An injection port with a septum allows the supplementation of the broth medium, for example, with neutralizers, antibiotic inactivators, or growth supplements.
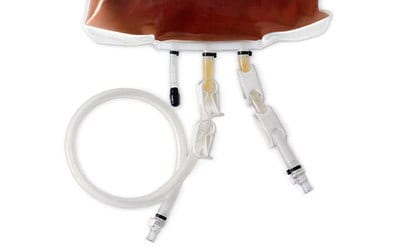
Connections of bag with injection port, outlet tubing, inlet tubing (from left to right).

Handle at top of 10 L ready-to-use bag for convenient use.

Tripple-bagged 10 L ready-to-use bag containing TSB in transport box
Maximize Safety and Accuracy Your Aseptic Process Simulations (APS)
With our reliable culture media, you can now ensure accuracy of your aseptic process simulations.
- True safety: Accurate and convenient APS
- Top standards: Internationally compliant quality — unconcerned by BSE
- Total confidence: Comprehensive documentation
Learn more about Aseptic Process Simulation Culture Media and best practices in our webinars: Media Selection Made Easy and Key Challenges When Selecting Media for Media Fill Tests.
True Safety: Accurate and Convenient Aseptic Simulation Process (APS)
False results due to low-quality culture media are a major concern in APS. With the robust validated process of our high-quality broths, you can eliminate media-related positives from your aseptic process simulation. Our stringent sterilization process inactivates all viable bacteria, yeast, mold, spores, and mycoplasma.
Our granulated and liquid media are both available as either Tryptic Soy Broth (TSB) or non-animal Vegetable Peptone Broth (VPB). Simply choose the format that suits your needs:
Dehydrated culture media: irradiated; highly soluble and filterable in low-dust granulated form; triple-wrapped for secure cleanroom use.
Ready-to-use liquid broth: Supplied triple-wrapped in gas impermeable bags for aseptic connections; direct link to production vessels and delivery systems.
Peptones source from BSE/TSE-free countries
Whether vegetable- or animal-based, every batch of our culture media is produced and tested in compliance with stringent international standards. For additional security, we source all concerned bovine ingredients exclusively from countries classified by the OIE as negligible risk countries.
- EU Note for Guidance (EMA/410/01 Rev. 03) aimed at minimizing the risk of infection from animal spongiform encephalopathy via pharmaceutical products.
- USDA standards for validation of aseptic preparations.
- All culture media with bovine peptones already comply with the future EU Directive on minimizing the risk of BSE from products of animal origin.
Total Confidence: Comprehensive Documentation
Detailed documentation is available for every culture medium we supply. Not only does this support your GMP aseptic process simulation, but it also simplifies your audits.
- BSE/TSE certificates provide full traceability of all animal-derived material and facilitate compliance with requirements.
- Quality agreements with suppliers ensure the consistent quality of our products.
- Certificates of Analysis (CoA) include detailed information about each product, such as actual results obtained from quality control testing.
Ready-to-use Culture Media Bags
如要继续阅读,请登录或创建帐户。
暂无帐户?