Hydrophobic Interaction Chromatography Setup
Column and media preparation
Equilibrate column with 5–10 column volumes of start buffer or until UV baseline and conductivity are stable.
Use prepacked columns to ensure the best performance and reproducible results. An evenly packed column ensures that component peaks are not unnecessarily broadened as sample passes down the column so that the best resolution can be achieved.
Allow buffers, media or prepacked columns to reach the same temperature before use. Rapid changes in temperature, for example, removing packed columns from a cold-room and then applying buffer at room temperature, can cause air bubbles in the packing and affect the separation.
Wash away storage solutions and/or preservatives before using any HIC medium. Wash columns with 10 column volumes of salt-free elution buffer before equilibrating with start buffer to avoid the risk of ethanol in the storage solution causing salt precipitation.
Appendix 2 gives details on column packing. The volume required for the packed bed is determined by the amount of sample to be purified and the binding capacity of the medium. Pack a column with approximately 5-fold excess of the binding capacity required (total protein should be equivalent to 20% of the binding capacity) and a bed height up to 20 cm.
Check column performance regularly by determining column efficiency and peak symmetry. See Appendix 2. Note that this does not apply to HiTrap™ columns.
Sample preparation
Simple steps to clarify any sample before application to a column will avoid the risk of blockage, reduce the need for stringent washing procedures and avoid deterioration in column performance and increases in back pressure. Appendix 1 contains a detailed overview of sample preparation techniques.
Correct sample preparation is essential in order to achieve optimal separation. In HIC, the initial binding conditions also influence the final selectivity of the separation.
Before starting any separation, establish the “salt stability window” for the sample, for example, add increasing amounts of salt to the crude sample in order to establish the concentration at which precipitation occurs. Ensure that the sample is below this salt concentration when applied to the column in order to avoid blockage. Refer to Appendix 1 for a guide to using ammonium sulfate in precipitation experiments.
When possible, test for biological activity of the target protein to establish the concentration range over which activity can be maintained (remember that high salt concentrations may need to be reduced before assaying for activity). Having established the salt stability window, begin with the highest salt concentration that retains biological activity and does not cause precipitation problems. The salt content and pH of the sample should be the same as those of the start buffer to ensure optimal binding conditions.
HIC requires a minimum of sample preparation work. Binding takes place predominantly as a result of the high salt conditions and the technique is fairly insensitive to pH conditions. It is not necessary to exchange the sample buffer before applying sample to a HIC column; simply ensure that there is sufficient salt, and adjust the pH directly, if necessary. Add salt from a high concentration stock solution to avoid the risk of precipitation due to local, high salt concentrations when salt is added as a solid.
- Samples must be clear and free from particulate matter, particularly when using media of particle sizes 34 μm or less. For small sample volumes, a syringe-tip filter of cellulose acetate or PVDF can be sufficient for sample filtration. Filter samples after the addition of salt and any other additives.
- Ensure that sample is at the same temperature as buffers, columns and chromatographic equipment.
- Use buffer exchange (see page 136) to remove chaotropic agents, such as guanidine hydrochloride or urea, that have been used for initial solubilization as they will inhibit hydrophobic interaction.
- If sample begins to precipitate at the salt concentration needed in the start buffer, reduce the salt concentration and divide the sample into smaller aliquots before application. The concentration of the start buffer is unchanged.
- Lipids or other very hydrophobic substances in the sample may interact with the medium, reducing binding capacity during the run and in subsequent runs. Using a slightly less hydrophobic medium (such as Butyl-S Sepharose® 6 Fast Flow) as a “pre-column” is one alternative for removing these contaminants before the main separation.
Concentration and viscosity
Viscosity varies with temperature and will increase at very high salt concentration. The solubility or viscosity of a sample may limit the quantity that can be applied to a column. High sample viscosity can cause an irregular flow pattern resulting in broad, distorted peaks and problems with backpressure. The critical parameter is the viscosity of the sample relative to the viscosity of the eluent.
- Dilute viscous samples. If dilution is not an option, using a lower salt concentration or a medium with a larger particle size may help to overcome viscosity problems. If high viscosity is caused by the presence of nucleic acid contaminants, see Appendix 1 for advice on their removal.
- Samples should generally not exceed 50 mg/ml protein, but may vary according to the type of sample and the type of chromatography medium.
Sample Application
Adjust the sample to the chosen salt concentration (and pH if necessary). Filter and apply to the column.
Wash with 5–10 column volumes of start buffer or until the UV baseline and conductivity are stable, indicating that all unbound material has washed through the column.
For efficient binding, the sample should be at the same salt concentration as the start buffer. The sample volume can be relatively large without affecting the separation since sample will bind near the top of the column as long as application conditions are correct.
- Apply samples directly to the column via a chromatography system, a peristaltic pump or a syringe. The choice of equipment depends largely on the sample volume, the size and type of column, the type of HIC medium and the requirements for gradient accuracy during elution.
Sample Load
Sample load (mass) is of greater importance than sample volume. The amount of sample that can be applied to a column depends on the binding capacity of the medium and the degree of resolution required. Binding capacity is determined largely by the medium, protein properties and the binding conditions, size and shape of the molecules, pore size of the matrix and, to a lesser extent by flow rate, temperature and pH.
Sample load has a major influence on resolution since the width of the peaks is directly related to the amount of substance present. In order to achieve satisfactory resolution, the total amount of protein applied and bound to the medium should not exceed the total binding capacity of the packed column.
- Apply up to 30% of the total binding capacity of the column for optimal resolution with gradient elution. Sample loads can be increased if resolution is satisfactory or when using a step elution.
Capacity can be increased, for example, by decreasing flow rates or optimizing start conditions to favor binding of the target protein(s) and minimize binding of contaminants.
Capacity will decrease with increasing flow rates so that a balance must be found between achieving the maximum dynamic binding capacity and a fast separation, particularly when applying large sample volumes.
Capacity will also decrease for molecules of very large diameter or length, for example, protein complexes >Mr 400 000, asymmetric proteins and DNA. These molecules are unable to penetrate the matrix pores, limiting their interaction primarily to the hydrophobic groups on the surface of the matrix. Since the exact distribution of pore sizes in some matrices can vary and the apparent size of a molecule can vary according to the buffer conditions, there is no distinct molecular weight cut-off point when molecules can or cannot penetrate the matrix pores.
Sample Volume
As a binding technique, HIC is independent of sample volume as long as the salt content of the sample and the start buffer ensure adequate binding conditions.
Temperature
It is generally agreed that the role played by temperature is highly complex. In practice this means that working at a constant temperature will improve reproducibility and that a separation developed at room temperature may not be reproducible under cold-room conditions or vice versa.
Figure 24 demonstrates the importance of having sample, start and elution buffers, columns and chromatographic equipment at the same temperature. Both separations were performed at room temperature (23 °C) under identical conditions except for the sample temperature (23 °C or 4 °C). With all components at the same temperature, the target protein was bound and then eluted in the middle of the gradient. With the sample at 4 °C, the target protein eluted in the flowthrough.
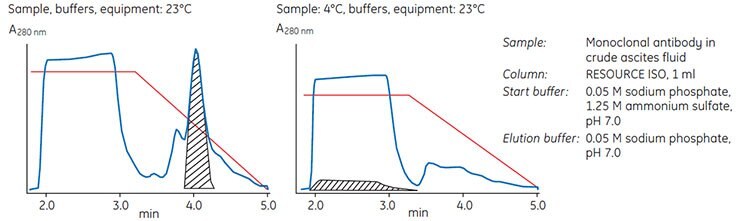
Figure 24.Influence of temperature on a HIC separation.
- In most cases, increasing temperature enhances hydrophobic interactions so working at lower temperatures (typically below 10 °C) can minimize aggregation caused by hydrophobic interactions between sample components. Lowering temperature can be used instead of adding detergents to improve solubility.
- Ensure that sample, column, start and elution buffers are at the same temperature. Note that temperature will also affect the viscosity of sample and buffers.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?