Duolink® PLA流式检测方案
本实验方案描述如何使用Duolink® PLA邻位连接试剂,通过流式细胞术检测细胞群内的单个蛋白质、蛋白修饰和蛋白相互。
材料和设备
Duolink® PLA试剂
Duolink® PLA的流式细胞术检测实验需要以下Duolink® PLA产品:
- Duolink® PLA探针(一对来自不同物种正负探针,与一抗宿主物种相匹配)。每个试剂盒包含:
- PLA探针(5x)— PLUS和/或MINUS,随产品而定
- 封闭溶液 — 用于在孵育抗体前封闭样品
- 抗体稀释剂 — 用于稀释PLA探针,而且如果需要,用于稀释一抗
注意:试剂盒所有组分4 °C储存。如有需要,可用Duolink® PLA Probemaker PLUS和/或Probemaker MINUS试剂盒制备定制的PLA探针。详情参见邻位探针制备指南。
- Duolink® flowPLA检测试剂(可选择绿色、橙色、红色和远红)。每个试剂盒包含:
- 连接原液(5x) – 稀释配制1X连接缓冲液
- 连接酶 (1 U/μL) – 用于配制连接液
- 扩增原液 (5x) – 稀释配制1X扩增缓冲液
- 聚合酶 (10 U/μL) - 用于配制扩增液
- 检测溶液(5x) – 稀释配制1X检测缓冲液
注意:所有组分-20 °C储存
注意:flowPLA检测试剂盒(DUO94001-DUO94005)提供的材料足够进行约40次反应,每次需100,000细胞溶于100 µL反应液中。还需要Duolink PLA探针(DUO92001—DUO92006、DUO92020和DUO92021)。
自备材料
- 检测目标蛋白质的一抗。当与Duolink® PLA探针配合使用时,必须用小鼠、兔子或山羊制备的。
- 超纯水(无菌过滤,Milli-Q®或类似水质)
- 悬浮细胞样本,已经过固定和透化方面的预处理
- Duolink®原位洗涤缓冲液(DUO82047)
- 1x PBS (P3813)
设备
- 流式细胞仪,搭配合适的滤光片和数据分析软件
- 37°C培养箱
- 冰块(用于酶)
- 移液器和吸头(1 μl至1000 μl)
- EP管、U形底或V形底96孔板
- 选配:96孔过滤板或过滤杯(Millipore UltraFree MC-HV),摇床
注意:用过滤板或过滤杯时,应采用建议的离心或真空参数。
DUOLINK® PLA流式方案
下列实验方案适用于检测溶于100 µL反应液的100,000个细胞 (1,000 cells / µL)。视需要,根据样本中的细胞数目调整体积。所有孵育均应在37 °C培养箱中进行。所有洗涤步骤均在室温进行。
试剂准备
- Duolink®洗涤缓冲液和1xPBS应在开始检测之前配制,将一小袋溶解在超纯水中,定容至1000 mL。1xPBS室温存放。洗涤缓冲液短期(短于两周)室温存放,长期则4 °C储存。
注意:使用前将溶液升至室温。
- 许多Duolink® PLA试剂以浓缩原液形式提供,应在临用前稀释。请勿储存稀释后的Duolink® PLA试剂。
Duolink® PLA实验方案
开始实验前,应先对悬浮细胞样本进行固定和透化预处理。可先固定、透化并用大量溶液封闭细胞,再分装到试管或板孔中用于Duolink® PLA染色,除非这些步骤需要独特的条件。
注意:Duolink®封闭液和抗体稀释剂随Duolink® PLA探针一起提供。如已有通过传统流式法优化获得的更能发挥一抗性能的溶液,可改用这类溶液。
- 封闭
- 固定和透化细胞后,离心并弃去洗涤缓冲液
- 按每µL细胞沉淀消耗10 µL Duolink®封闭液的用量滴液(1滴约30 µL)
- 37 °C培养箱孵育60分钟
- 一抗孵育
- 用Duolink®抗体稀释剂或适宜的抗体稀释剂将一抗稀释到适合浓度
- 在U底或V底微孔板每孔加入100,000细胞。
- 微孔板400 xg离心5分钟,弃去Duolink®封闭液。
- 每孔样本加入一抗溶液并混匀
- 按一抗最佳孵育温度和时间孵育
- Duolink® PLA探针孵育
- 用Duolink®抗体稀释剂1:5稀释PLUS和MINUS PLA探针。对于100 µL反应,取20 µL PLA探针MINUS原液,20 µL PLA探针PLUS原液和60 µL抗体稀释剂。为所有样品制备足量的溶液。
- 400 xg离心5分钟并弃去液体
- 每孔200 µL Duolink®洗涤缓冲液洗涤细胞2次,400 xg离心5分钟并弃去液体
- 加入PLA探针溶液并混匀
- 37 °C培养箱孵育1小时
- 连接
注意:连接酶要等到需要添加到样品中时再添加。确保连接缓冲液在使用前完全解冻并充分混合- 涡旋混匀5x Duolink®连接缓冲液
- 超纯水1:5稀释5x连接缓冲液并混匀。对于100 µL反应,将20 µL 5x连接缓冲液加到77.5 µL超纯水中。为所有样品制备足量的溶液。
- 400 xg离心5分钟并弃去液体
- 每孔200 µL Duolink®洗涤缓冲液洗涤细胞2次,400 xg离心5分钟并弃去液体
- 在洗涤过程中,使用冰块恢复从冰箱里取出的连接酶(-20 °C)
- 用1x连接缓冲液1:40稀释连接酶并混匀。对于100 µL连接液,将2.5 µL连接酶加到97.5 µL 1x连接缓冲液中
- 加入连接液并混匀
- 37 °C培养箱孵育30分钟
- 扩增
注意:聚合酶要等到需要添加到样品中时再添加。- 涡旋混匀5x Duolink®扩增缓冲液
- 超纯水1:5稀释5x扩增缓冲液并混匀。对于100 µL反应,将20 µL 5x扩增缓冲液加到78.75 µL超纯水中。为所有样品制备足量的溶液
- 400 xg离心5分钟并弃去液体
- 每孔200 µL Duolink®洗涤缓冲液洗涤细胞2次,400 xg离心5分钟并弃液
- 在洗涤过程中,使用冰块恢复从冰箱里取出的聚合酶(-20℃)
- 用1x扩增缓冲液1:80稀释聚合酶并混匀。对于100 µL扩增液,将1.25 µL聚合酶加到98.75 µL 1x扩增缓冲液中
- 加入扩增液并混匀
- 37 °C培养箱孵育100分钟
注意:低丰度的蛋白或蛋白互作检测可能需要扩增更长时间(最久可过夜)
- 检测
注意:Duolink®检测缓冲液对光敏感。需避光使用。- 涡旋混匀5x检测缓冲液
- 超纯水1:5稀释并混匀。对于100 µL反应,将20 µL 5x检测缓冲液加到80 µL超纯水中。为所有样品制备足量的溶液。
- 400 xg离心5分钟并弃液
- 每孔200 µL Duolink®洗涤缓冲液洗涤细胞2次,400 xg离心5分钟并弃液
- 加入检测液并混匀
- 37 °C培养箱孵育30分钟
注意:根据蛋白丰度或背景水平调整检测时间(10-60分钟)。
- 最终洗涤
- 400 xg离心5分钟并弃液
- 每孔200 µL Duolink®洗涤缓冲液洗涤细胞,400 xg离心5分钟并弃液
- 用1x PBS按约1,000 cells/µL重悬细胞
结果
流式细胞术参数设置
设置适当的仪器参数进行流式细胞分析。分析前,建议先用固定但未染色的细胞(没有经过Duolink® PLA步骤处理)优化每个细胞系对应的仪器参数。确定设置后,同一实验中的所有样品都应采用相同的参数。使用前向散射和侧向散射参数设门圈定固定细胞,排除细胞碎片。还可通过侧向散射的宽度/高度对面积参数排除双连体。
技术阴性对照应包括各剔除一种一抗和剔除全部一抗的对照分别可确定每种一抗和Duolink® PLA探针的非特异性结合。要点需知,阴性对照(例如,剔除一抗但保留PLA探针)的背景荧光可能会超过未染色的固定细胞。这种技术阴性对照(例如,剔除一抗但保留PLA探针)可用于确定Duolink® PLA荧光实验方案检测的基础荧光域值并适用所有实验样本。
流式细胞术分析:
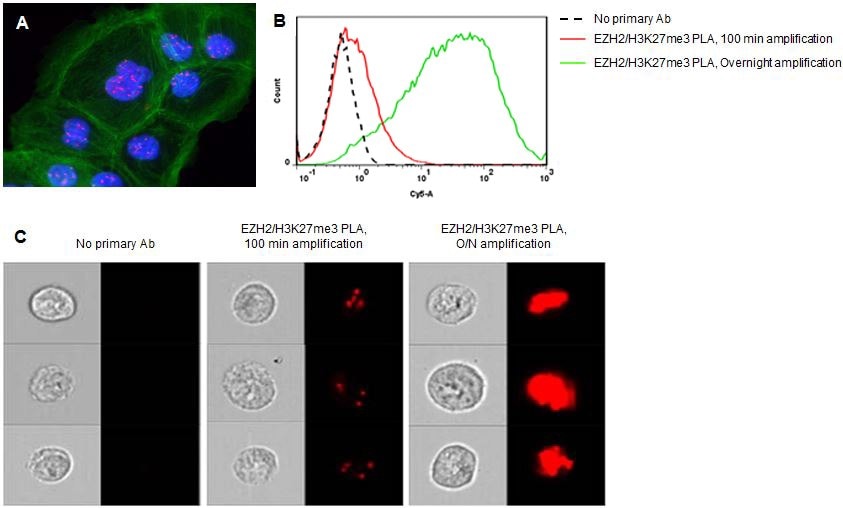
图 1.增加Duolink® PLA实验的扩增时间有助于流式检测低丰度蛋白靶标。通过Duolink® PLA实验检测组蛋白甲基转移酶EZH2介导的组蛋白3赖氨酸27的三甲基化(H3K27me3)。A)100分钟扩增后,荧光显微镜只检测到DU145细胞核(蓝色)中的少量PLA信号(红色)。FITC标记鬼笔环肽染色肌动蛋白(绿色)为复染。B)延长扩增时间后,对低丰度蛋白事件(例如EZH2-H3K27me3相互作用)的流式检测也随之增强。C)结合Duolink® PLA技术与成像流式细胞术可定位更大细胞群中的蛋白或蛋白事件(相互作用或修饰)。
快速实验指南
首先,需按照适合所选一抗的条件恰当固定和透化细胞样本。下列实验方案适用于检测100,000个细胞的样本,溶于100 µL反应液(1,000 cells / µL)。视需要,根据样本中的细胞数目调整体积。使细胞保持悬浮避免结团。
材料
参考文献
如要继续阅读,请登录或创建帐户。
暂无帐户?