Induced Pluripotent Stem Cell Culture Protocols
Introduction
Induced pluripotent stem cells (iPSCs) have the capacity to give rise to differentiated progeny representative of all three germ layers of the body: ectoderm, endoderm, and mesoderm. The ability to expand human iPSCs in vitro and subject them to cell-type specific differentiation protocols is critical for generating patient derived “disease-in-a-dish” cellular models for basic stem cell research and drug-discovery applications.
This protocol guide details steps on how to thaw, culture and cryopreserve human iPSCs supplied by the European Bank of induced pluripotent Stem Cells (EBiSC). Human induced pluripotent stem cell (iPSC) lines are different from any other established cell line. If you are not familiar with culturing iPSCs make sure you read the following instructions carefully.
Key Points for Success
- Read these instructions carefully before starting, including the sections on required reagents, thawing, passaging, precautions, and troubleshooting tips.
- Make sure all necessary reagents are available prior to thawing the cells.
- Use the correct media and matrix combination. iPSCs require specialized media and culture conditions.
- Make sure your equipment is calibrated regularly and no reagents have expired.
Methods
The certificate of analysis (CofA) makes recommendations for thawing cells in media and matrix that are specific to each cell line. Where required, the matrix and media used can be changed to an alternative during passaging only. Cells may require time to adapt following a change of media or matrix. No guarantees can be given regarding cell viability or quality when the recommended tissue culture system is not used.
Extracellular Matrix Preparation
Preparation of Basement Membrane Extract (BME)
Stem Cell Qualified ECM Gel Matrix (CC131) is a soluble basement membrane extract (BME) purified from Engelbreth-Holm-Swarm (EHS) tumors that has been pre-qualified for human ES/iPS cell culture. The matrix polymerizes at 20-40°C to form a reconstituted basement membrane. The product contains laminin, collagen IV, entactin and heparan sulfate among other ECMs. The matrix eliminates the need for time-consuming prescreening and lot identification, provides an optimized ECM coating necessary for long term culture of pluripotent human ES/iPS cells, and supports the culture of human ES/iPS cells in multiple serum-free/feeder free human ES/iPS cell expansion media (i.e. mTeSR®, PluriSTEM™). Below are general guidelines for the coating of 6-well plates using the Stem Cell Qualified ECM Gel Matrix. All procedures should be performed under aseptic conditions in a biological safety cabinet.
- Thaw the ECM Gel Matrix (CC131) in the 2-8°C fridge one day before use. Once thawed, maintain ECM Gel on ice at all times and use pre-cooled medium and pipettes to avoid gelling of the product.
- Dilute the ECM Gel Matrix (CC131) 1:80 with cold DMEM/F12 (D6421) medium. Scale up or down according to the volumes required. Below is an example for coating a 6-well plate with 1.5 mL of 1:80 diluted ECM Gel.
To make a 1:20 dilution, add 0.5 mL ECM Gel to 9.5 mL cold DMEM/F12 or DMEM medium into a 15 mL conical tube. Total volume = 10 mL.
A further 4-fold dilution is required to make the final 1:80 dilution. Add 2.5 mL of 1:20 diluted ECM Gel to 7.5 mL cold DMEM/F12 medium. Total volume = 10 mL
NOTE: The recommended dilution is 1:80, however more concentrated Stem Cell Qualified ECM Gel may be used if desired. - Add 1.5 mL of the 1:80 diluted ECM Gel Matrix (CC131) to each well of a 6-well plate. Swirl the culture plates to spread the ECM Gel Matrix evenly across the surface of the plate. Store in a 2 – 8°C fridge overnight or at least 2 hours in the fridge before use. If not used immediately, parafilm wrap the ECM coated culture plates and store at 2-8°C until ready to use. Use the ECM coated culture plates within 3-4 days.
- Prior to seeding the cells, bring the plate back to room temperature for 10-15 minutes, remove the coating solution and add 3 mL/well of human ES/iPSC growth media (SCM130). Cells can now be plated onto the newly coated plates.
IMPORTANT: Do not allow the plates to dry out.
Preparation of Vitronectin
- Upon receipt, store Vitronectin (CC130) at -80°C. Prior to use, thaw the stock vial of Vitronectin at room temperature and prepare 60 μL aliquots in sterile polypropylene tubes. Freeze the aliquots at -80°C or use immediately. One 60 μL aliquot is sufficient for coating all wells of a 6-well plate.
- To prepare Vitronectin at a working concentration of 0.5μg/cm2, dilute the Vitronectin 1:100 by gently mixing 6 mL of room temperature PBS (D8537) with 60 μL of Vitronectin. Add 1 mL of diluted Vitronectin to each well of a 6-well plate.
- Incubate the coated culture vessels at room temperature for 1 hour. If storage is required, vessels can be sealed with Parafilm® (P7793) and stored at 2-8°C for up to 3 days. Allow the vessel to equilibrate to room temperature for 1 hour prior to use.
- To prepare the vessel for culture, remove the excess Vitronectin from the culture vessel and discard. It is not necessary to wash the culture vessel after the removal of Vitronectin.
Cell Culture Media Preparation
mTeSR™-1 Media
- When required, remove the mTeSR™1 supplement (5x) from the freezer and thaw overnight at 2-8 °C prior to use. Do not thaw at 37 °C.
- Aseptically add 100 mL of mTeSR™1 supplement (5x) to 400 mL of cold (2-8 °C) basal medium.
- Aliquot medium into volumes required for 1 week of culture work.
- Complete mTeSR™1 may be stored at 2-8 °C for 1 week or at -20 °C for 6 months. Frozen complete mTeSR™1 may be thawed once. Do not repeatedly freeze thaw medium. Prior to use, warm mTeSR™1 to room temperature. Do not leave medium at room temperature for longer than 2 hours per day, and avoid exposure to light to avoid degradation of medium components.
Essential 8™ (TeSR™-E8) Media
- When required, remove the E8 supplement (50x) from the freezer and thaw overnight at 2-8°C prior to use. Do not thaw at 37°C.
- Aseptically remove 10 mL of E8 basal medium to leave 490 mL.
- Add 10 mL of E8 supplement (50x) to the 490 mL of basal cold (2-8 °C) medium.
- Aliquot medium into volumes required for 1 week of culture work.
- Complete E8 may be stored at 2-8 °C for 1 week or at -20 °C for 6 months. Frozen complete E8 may be thawed once. Do not repeatedly freeze thaw medium. Prior to use, warm E8 to room temperature. Do not leave medium at room temperature for longer than 2 hours per day and avoid exposure to light to avoid degradation of medium components.
PluriSTEM® Human ES/iPSC Media
PluriSTEM® medium (SCM130, SCM132) is a complete small molecule based serum-free medium that enables feeder-free culture of human ES/iPS cells and allows for media exchanges every other day without compromising morphology or long term functionality. The media is complete and does not require further supplementation. When required, remove the media from the freezer and thaw overnight at 2-8°C prior to use. Do not thaw at 37°C. Once thawed, PluriSTEM® media should be stored at 2-8°C and used within two weeks.
Thawing of Human iPSCs
- Cells should be thawed rapidly by placing the cryovial in a water bath set to maintain 37 °C. Swirl the cryovial gently in the water bath to ensure rapid thaw but do not submerge the cap of the cryovial. Disinfect the cryovial with 70% ethanol (793213) or an equivalent disinfectant before opening.
- Using a 5 mL sterile pipette, transfer the cryoprotectant/cells mix from the cryovial into a 15 mL centrifuge tube. Care should be taken not to physically damage cells.
- Slowly, drop by drop, add 10 ml of appropriate medium at room temperature to the cells in the 15 mL centrifuge tube. Gently rock the 15 mL centrifuge tube back and forth while adding drops of medium. This is a crucial step than minimizes osmotic shock to the cells and helps to ensure that cells are treated as gently as possible.
- Check tube to ensure all cell contents are removed. If needed, rinse with 1 mL of appropriate medium.
- A small amount of cells can be used for performing a cell count. A single cell suspension should be created using trypsin or other appropriate cell detachment medium. As a general guideline, the seeding density range for one well of a 6-well plate is between 2x105 - 1x106 viable cells. Refer to CofA for guidelines for any specific EBiSC cell line lot number.
- Centrifuge the cells at 200 x g for 2 minutes. Remove and discard the supernatant.
- Prepare culture vessels by adding an appropriate amount of medium (for example 1.5 – 2 mL per one well of a 6-well plate).
- Gently tap the 15 mL centrifuge tube to dislodge the cell pellet, then gently add 1 mL of appropriate medium and seed into 2 wells of a coated 6-well plate (adjust if using other culture formats or if advised differently in the Certificate of Analysis). Do not overaspirate the cells, as this will lead to decreased viability due to generation of a single cell suspension.
- Gently rock plate side to side, and back and forth to spread the cells evenly across the well.
- It is advisable to record images of cells immediately post-thaw, at 48 hours and at approximately 70-80% confluence.
Culturing of Human iPSCs
- It is good practice to observe iPSC lines daily under phase contrast microscopy (4x, 10x, 20x and 40x magnification) to check for iPSC-like morphology, the presence of differentiated cells and confluence. A typical scoring is outlined below:
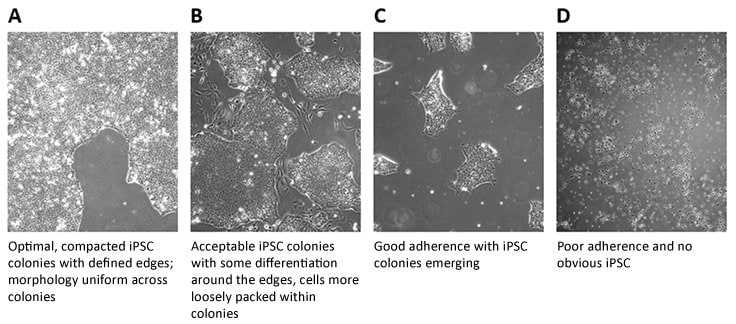
Figure 1.Scoring of iPSC Colonies
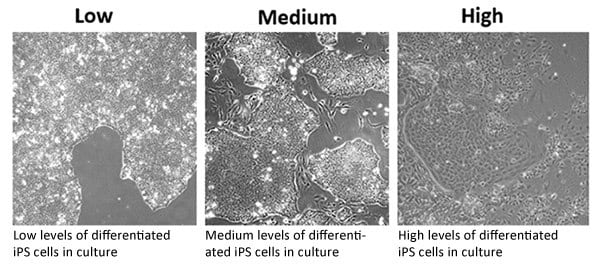
Figure 2.Differentiation Levels within iPSC Cultures
- Cells are fed by removing ~95% of the medium from the wells using an aspirator pipette. Do not completely remove the medium; a thin film of medium should cover the cell layer to avoid drying out the cells.
- Aseptically add 2 mL of fresh medium per 1 well of a 6-well plate by gently adding to the side of the well. Incubate cells at 37 °C/ 5% CO2.
- Typically, medium exchanges occur daily on six of seven days with increased volume of media (1.5x-2x the normal amount; cell density dependent) if cells need to be left for longer periods between media change. Do not exceed two days between medium exchanges.
Passaging of Human iPSCs
EZ-LiFT™ Reagent Passaging Protocol
- Warm EZ-LiFT™ Reagent (SCM139) to 37°C before starting.
- Aspirate the culture medium and wash wells twice with 1.5 mL EZ-LiFT™ Reagent (SCM139).
- Add 1 mL of EZ-LiFT™ reagent (SCM139) to each well. Incubate the plate at 37°C for 4 minutes.
- After 4 minutes, tap rapidly on the bottom of the plate (i.e. 20-25 taps in 5 secs).
- Place the plate back in the 37°C incubator for an additional 4 minutes.
- After 4 minutes, tap rapidly on the bottom of the plate (i.e. 20-25 taps in 5 secs).
- Perform a quick inspection of the well(s) by microscope.
1. If a significant number of detached clumps are visible, proceed to step 8.
2. If no obvious detachment is observed, repeat steps 4-7 except that in step 5, the 37°C incubation
should be for 2 instead of 4 minutes. Proceed to step 8. - Gently collect the cell suspension (~1 mL) and transfer to a 15 mL conical tube. Neutralize with 5 mL of culture medium by gently adding the medium to the cell suspension. Do not pipette up and down as this may break cell clumps into a single-cell suspension.
- Centrifuge at 800 rpm for 3 minutes. Aspirate the supernatant.
- Gently resuspend the cell pellet in 1 mL medium that supports pluripotency, such as PluriSTEM® (SCM130). Do not pipette up and down more than two times. Over-pipetting may result in single cell dissociation.
- Passage dissociated cell clumps to newly coated 6 well plates. Split ratio should be between 1:6 and 1:30. Monitor cells daily. Cells will typically reach 60-80% confluence in 6-8 days, depending upon the split ratio.
Accutase Passaging Protocol
- Aliquot sufficient PluriSTEM® (SCM130), Accutase (A6964) and DMEM/F12 (D6421) to passage the cells. Warm reagents at room temperature.
- One hour before the cells are to be passaged, add ROCK Inhibitor (ROCKi), Y-27632 (SCM075) to each well of the 6-well plate at a final concentration of 10 μM.
- After 1 hour, use a dissection microscope to visually inspect the plate containing human pluripotent cells to be passaged. Remove areas of spontaneous differentiation.
- Aspirate the medium containing the cells from the scraped areas from the well. Rinse with 2 mL per well of DMEM/F-12 (D6421) medium or 1X PBS (D8537).
- Aspirate and replace with 1 mL of Accutase (A6964) per well of a 6-well-plate. Incubate at 37°C for 8-10 minutes.
- Quench the Accutase reaction by adding 1 mL PluriSTEM® medium (SCM130) for each mL of Accutase used. Gently detach cells using a sterile 1000-μL pipette tip.
- Collect the dissociated cells to a 15 mL conical tube. Rinse the wells with an additional 2 mL of PluriSTEM® medium (SCM130) to collect any remaining cells. Add the rinse to the 15 mL conical tube.
- Centrifuge the 15 mL conical tube containing the cell suspension at 300 x g for 5 minutes at room temperature.
- Aspirate the supernatant. Resuspend the cells in fresh PluriSTEM® medium (SCM130) containing 10 μM ROCK Inhibitor, Y-27632 (SCM075).
- Count the number of cells using a Scepter™ cell counter or hemocytometer. Ensure that the cells are in a single cell suspension. Determine the cell viability using Trypan Blue (T8154) exclusion.
- Set up a titration of different cell densities ranging from 0.5 –1x104 cells/cm2. This corresponds to 50,000 – 100,000 cells per well of a Matrigel-coated 6-well plate in PluriSTEM® medium containing 10 μM ROCK Inhibitor.
- The next day, replace with fresh PluriSTEM® media (SCM130). Replace with fresh PluriSTEM® medium (SCM130) every 2 days (3 mL volume per well). Cells can be passaged every 5-7 days.
Dispase Passaging Protocol
- Aliquot sufficient Dispase II, 1 mg/mL (CC130) and DMEM/F12 (D6421) to passage the cells. Warm reagents at room temperature.
- Use a dissection microscope to visually inspect the plate containing human pluripotent cells to be passaged. Inspect the colonies for areas of spontaneous differentiation.
- Use a sterile p200 pipette tip attached to a p200 pipetman to scrape away areas of spontaneous differentiation.
- Aspirate the medium containing the cells from the scraped areas from the well. Rinse with 2 mL per well of DMEM/F-12 (D6421) medium or 1X PBS (D8537).
- Add 1 mL Dispase II, 1 mg/mL (CC130) per well of the 6-well plate containing pluripotent human ES or iPS cells to be passaged.
- Incubate at 37°C for 6-7 minutes. After incubation, visually inspect the colonies under a microscope. The edges of the colonies may appear slightly rounded up and/or folded away from the plate surface, but the majority of the colony should still be attached to the plate.
- Aspirate the Dispase II (CC130) and gently rinse each well two times with 2 mL 1X PBS (D8537) or DMEM/F12 (D6421) medium.
- Add 1.5–2 mL PluriSTEM® medium (SCM130) to each well. Gently detach the colonies using a cell scraper.
- Use a 5 mL serological pipette to collect the cell aggregates to a 15 mL conical tube. Minimize pipetting up and down as this may break up the colonies to suboptimal small pieces.
- Rinse the wells with an additional 2 mL of PluriSTEM® medium (SCM130) per well to collect any remaining cell aggregates. Add the rinse to the 15 mL conical tube.
- Centrifuge the 15 mL conical tube containing the cell aggregates at 300 x g for 5 minutes at room temperature.
- Aspirate the supernatant. Resuspend the cell aggregates in an appropriate volume of PluriSTEM® medium (SCM130) for passaging. Split the cells 1:3 to 1:6, depending on number of cell clumps.
- Place the plate in a 37°C incubator. Agitate the plate gently from side to side and forward and backwards to ensure that the cell aggregates are evenly distributed across the surface of the well.
- The next day, replace with 3 mL per well of fresh PluriSTEM® medium (SCM130). Cells can be passaged every 5-7 days.
Cryopreservation of Human iPSCs
- Keep reagents and freezing container (e.g. Mr. Frosty) chilled during the cryopreservation procedure.
- Cells must be cryopreserved when in their log phase of growth to enhance survival upon thaw. The optimal time for harvest is normally when cells are approximately 70-80% confluent
- The type of cryoprotectant medium used depends on culture conditions and laboratory preferences. Use either commercially available Cryostor® CS10 (C2874) or DMSO (D2650) based freeze mix (10% DMSO in FBS and culture medium). Cryostor® medium is supplied ready to use and is stored at 2-8°C. To prepare DMSO based cryoprotectant, mix 40% FBS with 10% DMSO, then mix with 50% appropriate medium.
- Remove spent medium from the tissue culture vessel and wash the vessel twice with the recommended volume of wash buffer depending on culture conditions (Wash buffer for Cultrex/Matrigel is 0.5mM EDTA; wash buffer for Vitronectin is PBS).
- To lift the cells from the tissue culture plastic, add 1 mL of 0.5mM EDTA (03690) to the tissue culture vessel. Incubate the cells for the recommended time and temperature, depending on matrix used. Aspirate the EDTA from the well. Care must be taken colonies will be very loosely attached to the plastic.
- Add 1 mL of cryoprotectant per well. Gently wash the cryoprotectant over the vessel with a 1 mL sterile pipette to dislodge the cells from the plastic. Do not aspirate more than 3 times, to avoid dissociating the cell clumps into single cells. Place the cryoprotectant and cell mix into an appropriately labelled cryovial.
- If cryopreservation of more wells is desired, cells from the same passage number and culture condition should be pooled together. An aliquot of pooled cells can be used for a cell count. Centrifuge harvested cells at 200xg for 2 minutes, aspirate spent medium and gently resuspend the cell pellet in an appropriate volume of cryoprotectant. One well of a 6-well plate gives rise to approximately 1-2 x 106 cells. It is recommended to freeze around 1-2 x 106 cells per cryovial. Use 1 mL of cryoprotectant-cell mix per cryovial.
- Immediately place the cryovials into a pre-chilled (2-8°C) Mr. Frosty tub, then immediately transfer the Mr. Frosty tub to a -80°C freezer. Allow the cells to remain at -80°C overnight (16-36 hours). Once frozen, transfer the cells, on dry ice, to an ultra-low temperature storage vessel (liquid introgen or -150°C freezer).
Characterization of Human iPSCs
The undifferentiated state of the iPSCs is characterized by a high level of expression of alkaline phosphatase (SCR004) and the stem cell transcription factors Nanog, Oct-4 and Sox-2. These cells also exhibit marked differences from their murine counterparts with regard to their expression of stage-specific embryonic antigen (SSEA1, 3, 4) and Podocalyxin (TRA-1-60, TRA-1-81) markers. Cells can be analyzed by antibody ICC staining using stem cell characterization kits (SCR001, SCR002, SCR078) or by PCR analysis.
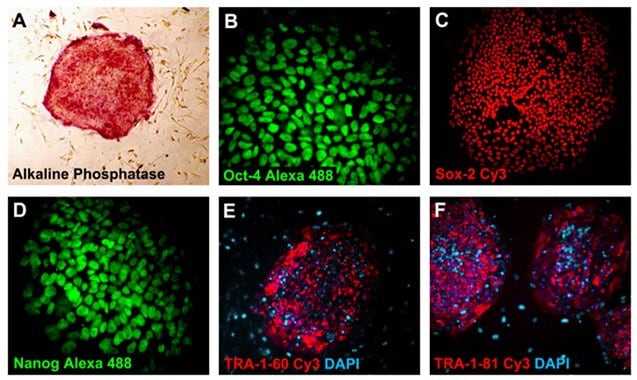
Figure 3. Pluripotent iPS cells express pluripotency markers. Alkaline phosphatase (40x) (A), Oct-4 Alexa 488 (MAB4401A4, 400x) (B), Sox-2 Cy3 (MAB4423C3, 100x) (C), Nanog Alexa 488 (MABD24A4, 400x) (D), TRA-1-60 Cy3 (MAB4360C3, 100x) (E), and TRA-1-81-Cy3 (MAB4381C3, 100x) (F). Nuclei were counterstained with DAPI (blue) (D9542).
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?