Fluorescent Multiplex Detection using Antibody Atto Dye Conjugates
Immunoblotting (Western blot transfer) is a common technique in modern proteomics research. After electrophoresis, proteins are immobilized on a nitrocellulose or PVDF membrane and then probed with a primary antibody against the protein-of-interest. A secondary antibody conjugated to horseradish peroxidase or alkaline phosphatase is subsequently bound to the primary antibody. A chemiluminescent enzyme substrate is added, and the resulting light is detected using a CCD camera or X-ray film exposure. Typically, immunoblot detection targets a single protein. In certain applications, however, such as the comparison of a protein in its phosphorylated and nonphosphorylated states, or the determination of the relative abundance of a protein of interest to a control protein (e.g., actin), multiplex detection on a single membrane is desirable.
We offer secondary antibodies covalently linked to brightly fluorescent Atto dyes. Immunoblot multiplexing can be performed by using two secondary antibodies conjugated to different Atto dyes with different excitation/emission wavelengths (Figure 1). Blotting membranes do not need to be stripped and reprobed for additional detection, reducing handling and minimizing sources of error.
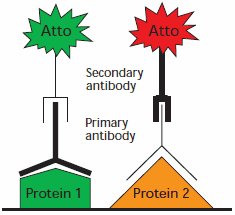
Figure 1. Schematic for multiplex application using two secondary antibodies with different Atto dyes.
Two-Color Multiplex Immunoblotting
In our first experiment, Protein 1 and Protein 2 (500 ng each) were separated on a 4-20% SDS-PAGE (Tris-Glycine) gel. The proteins were transferred to a low-fluorescence PVDF membrane and blocked using 5% BSA in PBS. The membrane was incubated with antibodies to Protein 1 and Protein 2 (1:1000 each).
Protein 1 was probed with a primary antibody developed in mouse, followed by goat anti-mouse-IgG-Atto 550 (Cat. No. 43394, diluted 1:1250) (λex = 532 nm, λem = 580 nm). Protein 2 was probed with a primary antibody developed in rabbit, followed by goat anti-rabbit-IgG-Atto 633 (Cat. No. 41176, diluted 1:1250) (λex = 633 nm, λem = 675 nm). Both proteins are detected on a single membrane, when run in separate lanes and when combined (Figure 2).
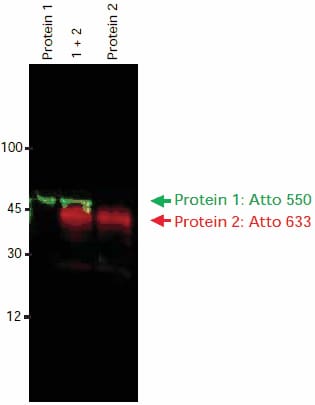
Figure 2. Immunoblot detection of Protein 1 and Protein 2 using two primary antibodies and two anti-IgG-Atto dye conjugates. Both secondary antibodies were developed in the same species (goat). Imaging was done sequentially using a FLA-3000 Fuji® laser scanner, first at an excitation wavelength of 532 nm with a 580 nm emission filter, then at an excitation wavelength of 633 nm with a 675 nm emission filter. The image overlay was done using a software tool.
Routine Immunoblotting using Atto Dye Conjugated Antibodies
Atto dye conjugated antibodies can also be used for routine immunoblotting. The limits of detection for amino-terminal FLAG-BAP™ fusion protein (Cat. No. P7582) using goat antimouse- IgG-Atto 647N (Cat. No. 50185) and goat anti-mouse- IgG-Atto 550 (Cat. No. 43394) conjugates are shown in separate experiments (Figure 3). FLAG-BAP protein (5 - 500 ng) was separated on a 4-20% SDS-PAGE gel. The protein was transferred to low-fluorescence PVDF membranes and blocked overnight using 5% BSA in PBS. The membranes were incubated with monoclonal ANTI-FLAG® M2 antibody (Cat. No. F1804, diluted 1:1000). One membrane was detected using goat anti-mouse- IgG-Atto 647N (1:1250) (λex = 633 nm, λem = 675 nm). The minimum amount of FLAG-BAP protein detected was 5 ng. A second membrane was detected using goat anti-mouse-IgG-Atto 550 (1:1250) (λex = 532 nm, λem = 580 nm). The minimum amount of FLAG-BAP protein detected was 20 ng. For the detection, we used a 532 nm laser combined with a 580 nm emission filter for Atto 550, and a 633 nm laser with a 675 nm emission filter for Atto 647N. Other imaging systems are possible with the corresponding excitation sources and emission filter settings.
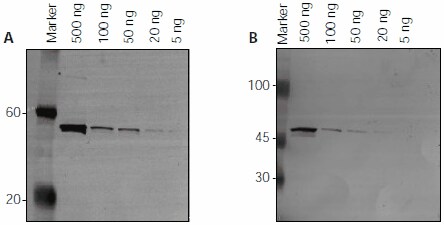
Figure 3. Detection of FLAG-BAP protein (500 ng – 5 ng) by immunoblotting using Atto dye labeled antibodies.
Image A: Antibody used was goat anti-mouse-IgG-Atto 647N. Imaging was performed at an excitation wavelength of 633 nm with a 675 nm emission filter.
Image B: Antibody used was goat anti-mouse-IgG-Atto 550. Imaging was performed at an excitation wavelength of 532 nm with a 580 nm emission filter. Imaging was performed on a FLA-3000 Fuji laser scanner. See text for further experimental details.
Additional Recommendations
- Incubation with the primary antibodies can be done sequentially without pooling, so that the antibodies can be reused.
- In order to avoid cross-reactivity, primary antibodies must be derived from different hosts, and secondary antibodies must be derived from the same host.
- low-fluorescence PVDF membrane such as ImmobilonTM-FL must be used to avoid high background.
- Drying the membranes enhances the signal intensity.
Atto-labeled antibodies offer new possibilities for immunoblot detection without enzymatic substrates as well as for single membrane multiplexing applications.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?