C-H官能团化_化学合成方法_有机化学-默克生命科学
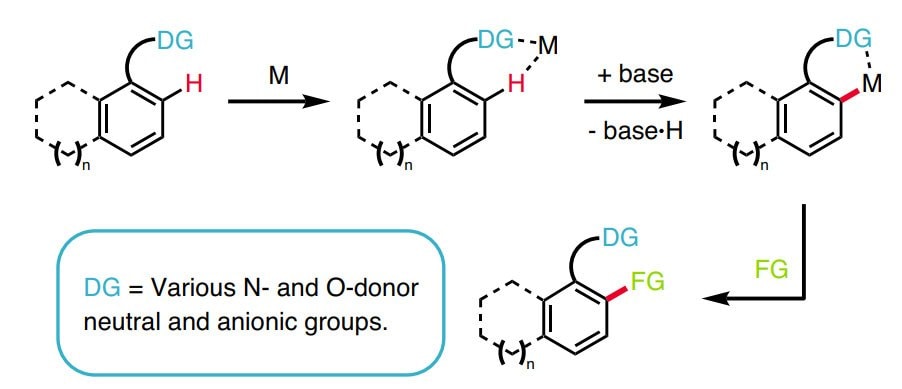
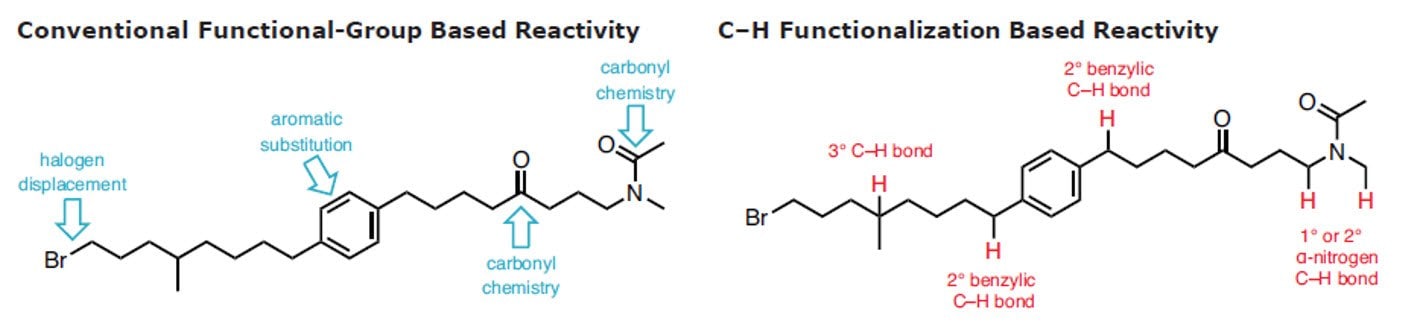
C-H官能团化被称为合成有机化学的最高目标。1 在有机化学、有机金属和催化领域的近期努力在了解C-H键的反应性和利用该洞见形成稳健反应方面取得了重大收获。这表明,是时候广泛地将这些策略引入逆合成字典中。2-11 以选择的、可控的方式将C–H可靠、可预测地转换为C–C、 C–N、 C–O 或 C–X 键有利于步骤经济和减少浪费。
推荐类别
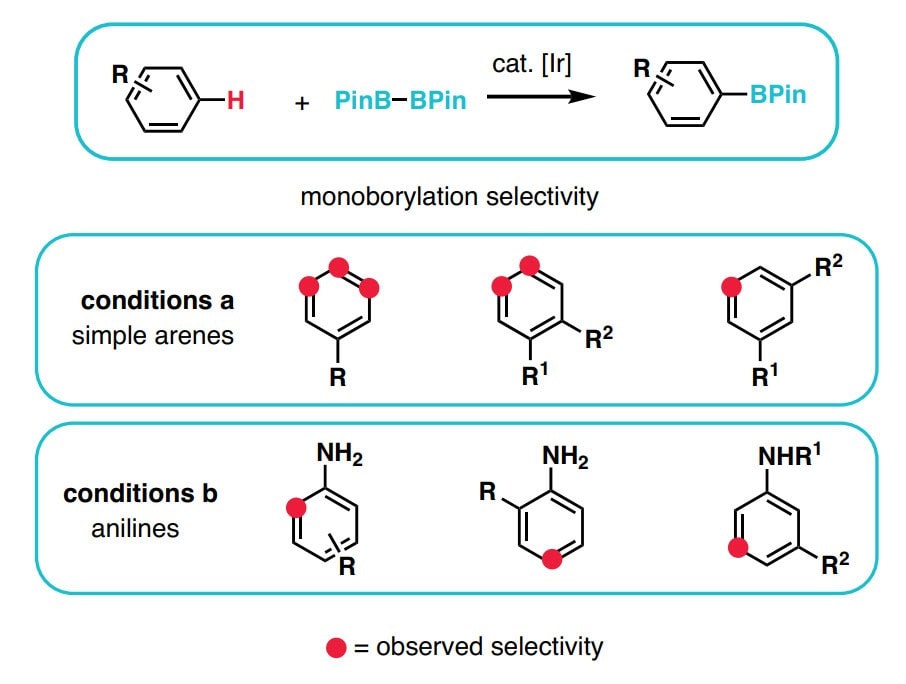
用于C–H活化的新方法扩展了给定分子中可靶向的位点数量,从而增加了将其精细化为更复杂产品的机会。此外,它还可实现在有机合成中靶向完全不同类型的化学键,尤其是具有高化学选择性的化学键。通过结合传统的官能团化学,C-H官能团化极大简化了用于构建复杂天然产物和药物化合物的化学合成过程。尽管C-H官能团化具有明显的优势,12但许多有机化学课程尚未将这种方法更新进去,更多进一步的信息可在C-H官能团化手册中找到。
相关文章
- Professor Karl Anker Jørgensen and his group have developed ethers which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
- Rapidly diversify (hetero)aromatic scaffolds for chemical industry needs amid resource and time constraints, ensuring efficiency.
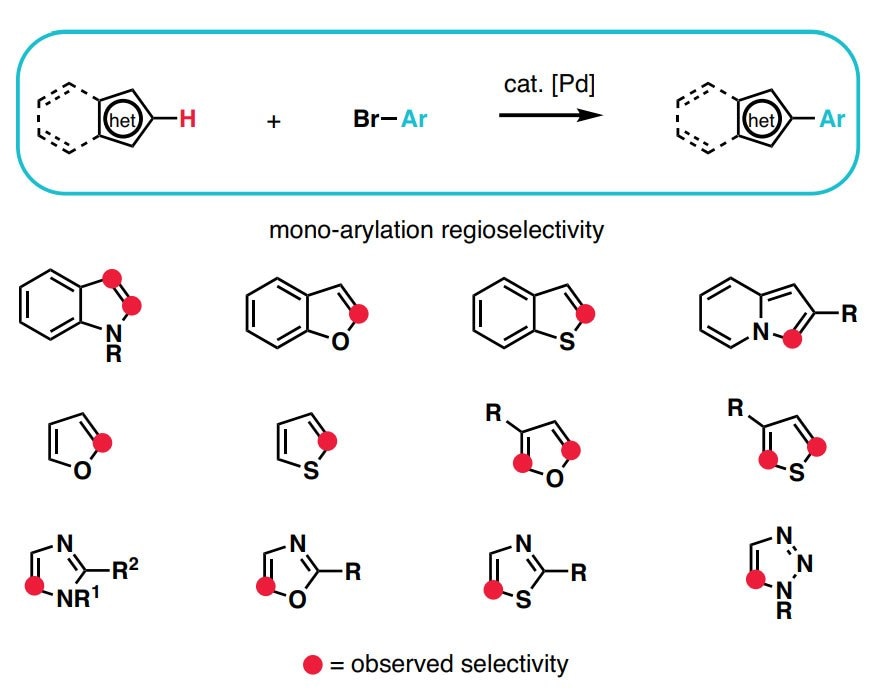
- Stanford's Du Bois group advances Rh-catalyzed C–H amination, producing heteroatom motifs in ring heterocycles.
- Aryl chlorides are commonly used in cross-coupling reactions and can serve as key intermediates towards the synthesis of pharmaceutical drug candidates and natural products.
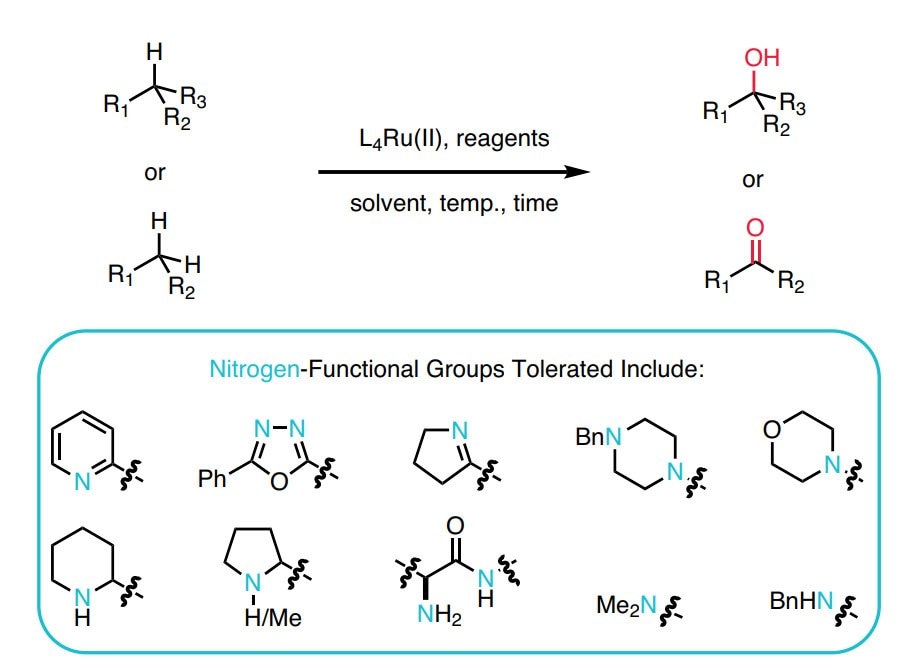
- A recyclable, ligand-free ruthenium catalyst for C–H activation reactions and concomitant C–C bond formation in the presence of water.
- 查看完整内容 (12)
相关实验方案
- Tips and troubleshooting for FFPE and frozen tissue immunohistochemistry (IHC) protocols using both brightfield analysis of chromogenic detection and fluorescent microscopy.
- 查看完整内容 (0)
我们可以提供哪些帮助
如有任何疑问,请提交客户支持请求
或联系我们的客户服务团队:
发送电子邮件至 custserv@sial.com
或致电 +1 (800) 244-1173
更多支持
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- 计算器与应用_缓冲液计算器_HPLC方法转换计算器-默克生命科学
默克该工具箱包括用于化学、生命科学、材料科学等方面的科学研究工具和资源。
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
参考文献
如要继续阅读,请登录或创建帐户。
暂无帐户?