登录 查看组织和合同定价。
选择尺寸
关于此项目
经验公式(希尔记法):
C17H18FN
化学文摘社编号:
分子量:
255.33
UNSPSC Code:
12352005
PubChem Substance ID:
MDL number:
InChI
1S/C17H18FN/c18-17(16-12-7-13-19-16,14-8-3-1-4-9-14)15-10-5-2-6-11-15/h1-6,8-11,16,19H,7,12-13H2/t16-/m0/s1
SMILES string
FC([C@@H]1CCCN1)(c2ccccc2)c3ccccc3
InChI key
PGKMVPFJFKOUDA-INIZCTEOSA-N
assay
97%
optical activity
[α]/D -27°, c = 1 in chloroform
optical purity
ee: 98% (HPLC)
refractive index
n20/D 1.576 (lit.)
density
1.096 g/mL at 25 °C (lit.)
Application
Features:
Catalyst for the enantioselective Weitz-Scheffer epoxidation of α,β-unsaturated aldehydes.
Condensation of this secondary amine with an aldehyde reversibly generates a β-fluoroiminium species where the fluorine atom is positioned gauche to the electropositive nitrogen centre.
Consequently, the phenyl substituents on the fluorine bearing carbon are placed in a predictable region of space. One of the groups effectively shields the upper face of the pendant iminium chain.
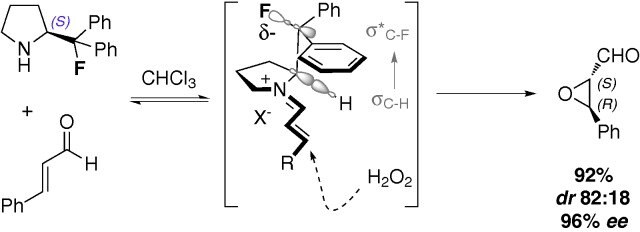 Addition of hydrogen peroxide occurs in a highly selective manner to furnish optically enriched epoxides with excellent levels of enantiocontrol (Scheme 1).
Addition of hydrogen peroxide occurs in a highly selective manner to furnish optically enriched epoxides with excellent levels of enantiocontrol (Scheme 1).
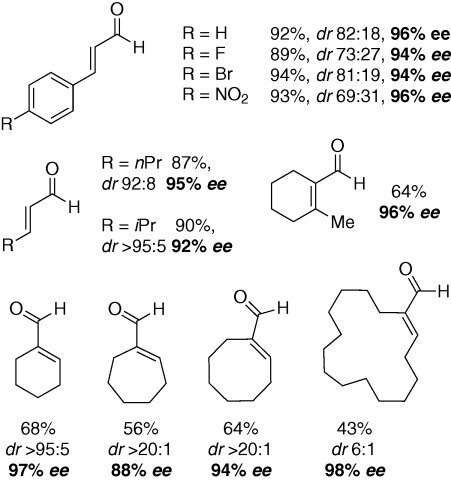 Simple α,β-disubstituted enals such as trans-cinnamaldehyde are smoothly converted to the corresponding epoxy aldehydes (up to 96% ee) as are their aliphatic analogues. The enantioselective, catalytic epoxidation of more challenging cyclic α,α,β-trisubstituted enals, and even an example of a α,α,β,β-tetrasubstituted enal, proceed with good yields and excellent enantioselectivities (up to 98% ee) (Scheme 2).
Simple α,β-disubstituted enals such as trans-cinnamaldehyde are smoothly converted to the corresponding epoxy aldehydes (up to 96% ee) as are their aliphatic analogues. The enantioselective, catalytic epoxidation of more challenging cyclic α,α,β-trisubstituted enals, and even an example of a α,α,β,β-tetrasubstituted enal, proceed with good yields and excellent enantioselectivities (up to 98% ee) (Scheme 2).
- Highly efficient
- Excellent enantioselectivities
- Available in both enantiomers
- Operationally simple transformations
Catalyst for the enantioselective Weitz-Scheffer epoxidation of α,β-unsaturated aldehydes.
Condensation of this secondary amine with an aldehyde reversibly generates a β-fluoroiminium species where the fluorine atom is positioned gauche to the electropositive nitrogen centre.
Consequently, the phenyl substituents on the fluorine bearing carbon are placed in a predictable region of space. One of the groups effectively shields the upper face of the pendant iminium chain.
 Addition of hydrogen peroxide occurs in a highly selective manner to furnish optically enriched epoxides with excellent levels of enantiocontrol (Scheme 1).
Addition of hydrogen peroxide occurs in a highly selective manner to furnish optically enriched epoxides with excellent levels of enantiocontrol (Scheme 1). Simple α,β-disubstituted enals such as trans-cinnamaldehyde are smoothly converted to the corresponding epoxy aldehydes (up to 96% ee) as are their aliphatic analogues. The enantioselective, catalytic epoxidation of more challenging cyclic α,α,β-trisubstituted enals, and even an example of a α,α,β,β-tetrasubstituted enal, proceed with good yields and excellent enantioselectivities (up to 98% ee) (Scheme 2).
Simple α,β-disubstituted enals such as trans-cinnamaldehyde are smoothly converted to the corresponding epoxy aldehydes (up to 96% ee) as are their aliphatic analogues. The enantioselective, catalytic epoxidation of more challenging cyclic α,α,β-trisubstituted enals, and even an example of a α,α,β,β-tetrasubstituted enal, proceed with good yields and excellent enantioselectivities (up to 98% ee) (Scheme 2).Legal Information
英国 Onyx Scientific 产品
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
存储类别
10 - Combustible liquids
wgk
WGK 3
flash_point_f
147.0 °F - closed cup
flash_point_c
63.9 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
法规信息
新产品
此项目有
Sparr, C.; et al.
Angewandte Chemie (International Edition in English), 121, 3065-3068 (2009)
Eva-Maria Tanzer et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(36), 11334-11342 (2012-07-26)
The fluorine-iminium ion gauche effect is triggered upon union of a secondary β-fluoroamine and an α,β-unsaturated aldehyde, providing a useful strategy for controlling the molecular topology of intermediates that are central to organocatalytic processes. The β-fluoroamine (S)-2-(fluorodiphenylmethyl)pyrrolidine (1) is an
O'Hagan, D.; et al.
Tetrahedron Asymmetry, 11, 2033-2036 (2000)
Zimmer, L. E.; et al.
Angewandte Chemie (International Edition in English), 123, 12062-12074 (2011)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持