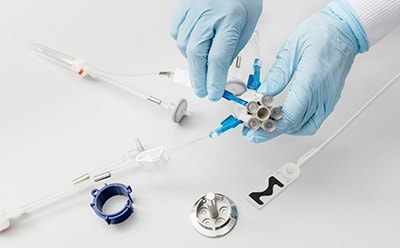
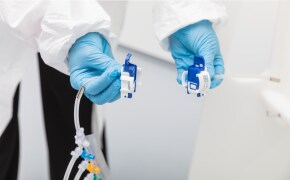
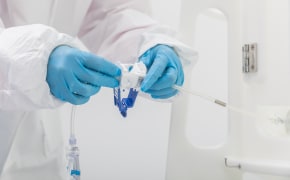
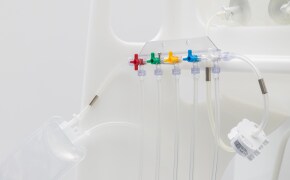
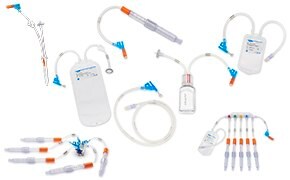
无菌采样
可靠的取样方法是实现稳定、高质量产出的有力保障。在制药和生物制药过程中实施无菌和无菌采样解决方案,可最大限度地降低污染风险,并实现准确、有代表性的样品采集,从而实现可靠的过程控制。我们提供的产品包括创新的手动无菌和自动无菌采样解决方案,旨在提高流程中任何步骤的效率和可靠性。
手动无菌取样
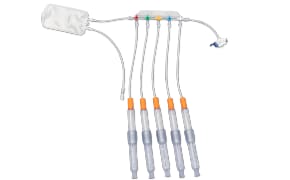
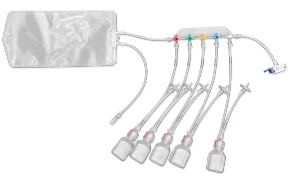
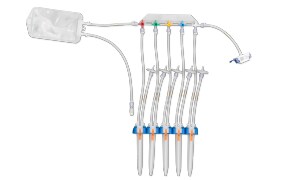
封闭式无菌取样为您提供生产过程中取样所需的安全性。NovaSeptum® GO无菌采样系统和NovaSeptum® SURe无菌采样组件是封闭式采样解决方案,可显著降低交叉污染的风险,并在整个过程中为您提供具有代表性的样品。解决方案包括用于一次性使用制造工艺的 NovaSeptum® SURe 无菌采样组件,或 NovaSeptum® GO 无菌采样系统,设计用于连接任何不锈钢单元操作。
- 封闭式设计: 保护过程和样品的完整性
- 易于使用: 即插即用选项,可选择连接方式和一系列容器
探索 NovaSeptum SURe assemblies for single-use processes
Explore NovaSeptum® 用于不锈钢或多用途工艺的 GO 系统
自动无菌采样
自动采样系统有助于在上游和下游工艺中以最高的效率和最少的操作员参与进行频繁的测量。我们的 MAST® 自动采样解决方案可实现在线、近乎实时的分析,支持全面的工艺理解和改进的工艺控制。
- 高效: 增加采样频率、缩短周转时间并最大限度减少操作员接触点
- 可靠: 确保快速样品转移和可追溯性,同时保持样品源无菌
Products
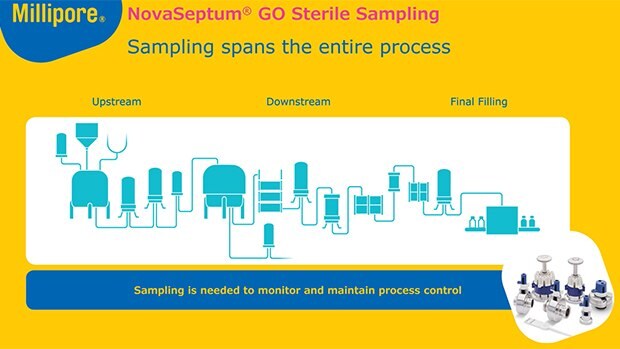
无菌采样适用于抗生素制造过程的每一个步骤
从生物反应器到最终灌装,NovaSeptum®无菌采样为生物制造商提供了一致且具有代表性的样品,用于分析 pH 值、电导率、细胞活力、代谢物、监控生物负载和内毒素水平。
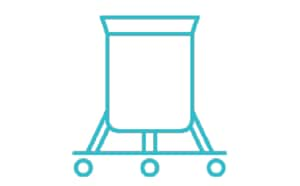
通过对 pH 值、渗透压和/或电导率进行采样,监测和调整工艺参数。
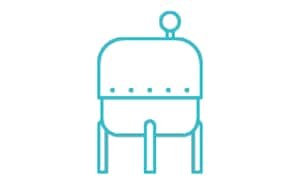
实现无菌流体添加,以调整或改进工艺。
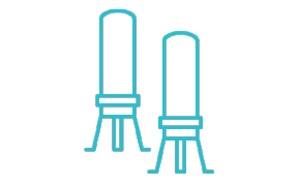
从所有采样容器中都能出色地回收微生物,采用在线连接支架,可轻松添加其他生物负载监测步骤。
在进行下一步加工之前,轻松监测生物负载、内毒素和质量,保护昂贵的树脂免受污染。
采样以证明无菌过滤的有效性,经过验证的采样容器可确保不影响内毒素的回收,最大限度地减少有价值产品的采样量。
与最终填充流程无缝集成、高度安全、具有代表性的样本。
要为您的工艺选择最佳一次性采样解决方案,首先要考虑您偏好的连接类型:热焊或无菌连接。然后通过 C-Flex® 软管或 AsepticQuik® 连接器将预先配置好的组件轻松集成到您的工艺中。
然后根据样品量、检测类型、储存条件和取样计划中的其他关键参数确定合适的取样容器。我们的 NovaSeptum® SURe 无菌采样组件是由三个或五个容器组成的标准单体和多体装置,有袋装、瓶装、注射器或锥形管装。
选择适合您工艺的夹头:Tri-clamp (TC)、In-line 或 In-Gold®。TC 夹头还可预装各种容器类型
。从一系列瓶子、袋子、注射器和锥形管中进行选择。这些产品有各种尺寸和配置--单次取样、分流或预装。
确保整个过程无菌。使用 Novaseptum® 手动压接工具断开样品连接。
为了最大限度地方便使用,我们提供了各种附件,例如包架、端口插头和安全标签。

MAST®自动采样解决方案是一种先进的自动无菌采样工具,旨在提高生物制药过程的效率和控制。通过将样品从多个来源直接采集并运送到不同的分析目的地,这一多功能解决方案加快了周转时间,减少了操作员接触点,并最大限度地降低了污染风险。它可与其他工艺分析技术 (PAT) 工具无缝集成,实现全面监控,并可根据各种工艺和规模进行配置。作为 GMP 就绪型解决方案,它可确保符合严格的法规要求,同时提供关键工艺参数的在线近实时分析和高效的资源利用。
。相关视频
相关产品资源
- White Paper: Aseptic Process Sampling Risk Mitigation – A Regulatory Perspective
There are significant consequences associated with microbial contamination during biopharmaceutical manufacturing. Contamination increases risks for the operator, the company, and potentially the patient, all of which can result in significant negative impact.
- Application Note: Microbial Integrity of NovaSeptum® Sampling Systems upon Performance of Multiple Sampling Actuations
The NovaSeptum® and NovaSeptum® GO sampling systems are a family of products designed for singleuse sterile sampling throughout the biomanufacturing process.
- User Guide: NovaSeptum® and NovaSeptum® GO Sampling Units
This user guide provides installation and use instructions
- Datasheet: NovaSeptum® GO Sterile Sampling System
To demonstrate the safety and integrity of your product, you need a standard-setting sampling solution that provides the flexibility to sample throughout your entire process. I
- Datasheet: NovaSeptum® SURe Sterile Sampling Assemblies
NovaSeptum® SURe single-use sterile sampling assemblies are designed to protect your single-use process from potential cross-contamination ensuring representative samples.
- Brochure: NovaSeptum® GO Sterile Sampling System
Continuing to set the sampling standard, our NovaSeptum® GO sterile sampling system is equipped with additional features for even safer sampling throughout your entire process.
- Technical article: Enhance Bioprocess Sampling with MAST® Autosampling Solution
Explore the MAST® Autosampling Solution for efficient, automated sampling in bioprocesses, supporting higher measurement frequency and reduced process risk.
- Datasheet: MAST® Autosampling Solution
The MAST® Autosampling Solution is a versatile & modality-agnostic aseptic autosampling solution for reliable on-line and near real-time sample analysis.
- White Paper: Automated Aseptic Sampling for Accelerated Access to Process and Quality Data in Upstream Bioprocessing
This white paper focuses on PAT and the use of automated sampling technology to accelerate analytical and quality control methods and provide an approach for access to in-line data to monitor processes in real time.
- Application Note: Enabling Accelerated Raman Model Calibration for Seamless and Reproducible Real-Time Monitoring by Combining Raman and Automated Sampling Technologies
Explore how automated sampling can accelerate the integration of a Raman analyzer for a bioreactor application, speeding up the Raman model building phase, and facilitating in-line, real-time monitoring of critical process parameters (CPPs) and critical quality attributes (CQAs) during the manufacturing process.
- White Paper: Accelerate Process Development with Automated Aseptic Sampling
This whitepaper describes evaluation of the MAST® Autosampling Solution as part of an automated PAT system implemented by Takeda Pharmaceuticals.
如要继续阅读,请登录或创建帐户。
暂无帐户?