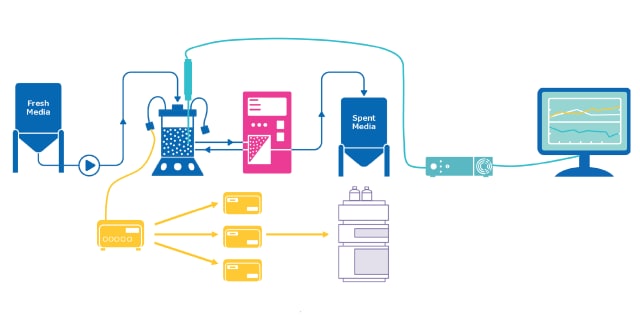
过程分析技术(PAT)通过实时监测和控制生产过程,确保生物制药生产的质量。该技术运用分析工具开发制造工艺,以适应物料和设备的变异性。 在识别出影响关键质量属性(CQAs)的关键工艺参数(CPPs)后,通过分析方法对CPPs进行监测与控制,使其维持在预设的设计空间内。这种方法将质量源于设计(QbD)原则融入生产过程,而非仅依赖最终产品检测。
生物制药中的实时监测与控制

PAT技术助力未来工厂建设
将PAT技术融入生产流程,为"生物加工4.0"及未来工厂奠定基础——这意味着通过实时监测、控制系统与数据分析实现制药生产的全面数字化转型。PAT技术赋能实时工艺监控,带来更深入的工艺理解、更强的敏捷性与灵活性,并提升质量保障水平。
分析洞察力提升工艺理解与产品质量
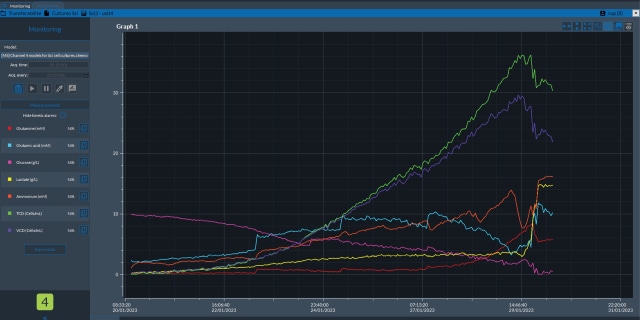
近年来,过程分析技术(PAT)通常采用色谱、光谱和/或质谱传感器,将其集成于上游和下游单元操作中。这些技术通过在线、联线或旁线方式应用,实现对工艺的实时监测与控制。通过提供实时洞察,这些传感器支持及时调整、优化和干预,最终提升对工艺的理解并改善产品质量。
相关分类
我们的现成可定制生物加工细胞培养基(CCM)产品,可提升单克隆抗体、疫苗、基因/细胞疗法等上游工艺的生产效率。
Mobius®生物反应器系列包括台式规模(2毫升和3升)、中试规模、临床规模以及...
使用Cellicon®细胞保留溶液,使灌注过程能够实现更高的细胞密度。
相关资源
- White Paper: Workforce 4.0: The Long-Term Success of the Biopharmaceutical Industry
A public-private approach to workforce 4.0 development, transitioning from conventional factories to “smart” manufacturing facilities.
- Application Note: In-Line Monitoring of CPPs and Capsid Titer in Upstream AAV Process with ProCellics™ Raman Analyzer
Explore the ProCellics™ Raman Analyzer for in-line monitoring of critical process parameters (CPPs) and capsid titer during AAV production in Human Embryonic Kidney (HEK) cell bioreactors.
- Application Note: In-line Real-time Monitoring of CHO Cell Culture Process Parameters Using Raman Spectroscopy
Cell culture processes are complex and highly variable and yet only a handful of key parameters such as temperature, pH, and dissolved oxygen (DO) are typically controlled in real time.
- Application Note: Implementation of Raman Spectroscopy for In-line Monitoring of CPPs of CHO Cell Perfusion Cultures
This application note introduces a case study for the implementation of a Raman spectroscopy soft-sensor for in-line and real-time monitoring of critical process parameters (CPP) in mammalian perfusion cell cultures.
- Application Note: Seamless Integration of Glucose Control Using Raman Spectroscopy in CHO Cell Cultures
Process analytical technology (PAT) and quality by design (QbD) are used in the biopharmaceutical industry to ensure quality is designed into a process and to achieve innovative quality improvements.
- Article: Influence of Cell Specific Parameters in a Dielectric Spectroscopy Conversion Model Used to Monitor Viable Cell Density in Bioreactors
In the biopharmaceutical industry, the use of mammalian cells to produce therapeutic proteins is becoming increasingly widespread. Monitoring of these cultures via different analysis techniques is essential to ensure a good quality product while respecting good manufacturing practice (GMP) regulations.
- White Paper: Automated Aseptic Sampling for Accelerated Access to Process and Quality Data in Upstream Bioprocessing
The biopharmaceutical market is experiencing increased demand for new medicines, reduction of costs, and new product classes, which is driving the need for increased flexibility and cost control measures in manufacturing.
- Data Sheet: MAST® Autosampling Solution
Current off-line sampling methods often present challenges related to sample source contamination, inadequate sample traceability and delivery, lengthy experimental turnaround times, and inconsistent results.
- Brochure: Process Development and Drug Manufacturing: Support Services
We provide comprehensive services for drug development and manufacturing, including technical and regulatory expertise and process development support.
咨询PAT专家
BioContinuum™平台:助您打造未来生物制造设施的利器!

如要继续阅读,请登录或创建帐户。
暂无帐户?