Cation Exchange Chromatography Membrane for mAb Purification
Cation exchange (CEX) chromatography is routinely used to remove host cell proteins (HCPs), aggregates, and viral particles from monoclonal antibody (mAb) feed streams that are not removed during primary capture using Protein A chromatography. CEX can be a significant bottleneck in the downstream process, limiting productivity (g/L/hr) due to the bind and elute mode of operation at slow flow rates with long residence times (4-6 minutes).
Downstream productivity can be increased with the use of membrane chromatography devices that operate at a much higher flow rate than CEX resins. Commercially available conventional membrane chromatography has had lower binding capacities than resin, which has limited its utility.
Natrix® CH Chromatography Membrane Offers High Binding Capacity
Natrix® CH devices contain a hydrogel single-use chromatography membrane with strong cation exchange (sulfonate) and hydrophobic (t-butyl) interaction components. It combines the best features of resin beads and traditional membranes to achieve high binding capacity at fast flow rates (Figure 1). Compared to resin beads, which rely on slow diffusive mass transfer, Natrix® CH chromatography membrane devices use convective mass transfer to achieve high binding rates and are ideal for intensified mAb processes, where speed and flexibility are critical.
Natrix® CH chromatography membrane devices can be operated in frontal or bind and elute modes at flow rates up to 10 MV/min (6 seconds residence time; 40x faster than CEX resins) without loss of binding capacity or impurity clearance regardless of operational mode. These high flow rates markedly improve productivity. Additional efficiencies can be achieved when operating in a frontal mode as high salt elution buffers, typically required in bind and elute CEX chromatography steps, are eliminated.
Figure 1.Natrix® proprietary technology enables a bead-like binding capacity with >20x shorter residence time. The unique 3D porous structure maximizes ligand density and eliminates diffusive transport speed limitations.
In the study described below, aggregate and HCP removal from a mAb feed stream using a Natrix® CH chromatography membrane device was compared with two conventional CEX membrane adsorbers. The purification performance of the Natrix® CH Micro device was also evaluated across twenty consecutive purification cycles.
Increased Dynamic Binding Capacity
The dynamic binding capacity (DBC) of the Natrix® CH chromatography membrane device was compared to that of conventional membrane adsorbers using feeds containing either lysozyme (3 g/L) - A or a mAb (3 g/L, in 50 mM acetate, pH 5.0 ) - B. The feed streams were loaded onto the devices at a flow rate of 10 MV/min until absorbance at 280 nm reached 10% breakthrough of the absorbance of the feed.
Figure 2 shows that the mAb DBC of Natrix® CH Micro was more than five times higher compared to conventional membrane adsorbers.
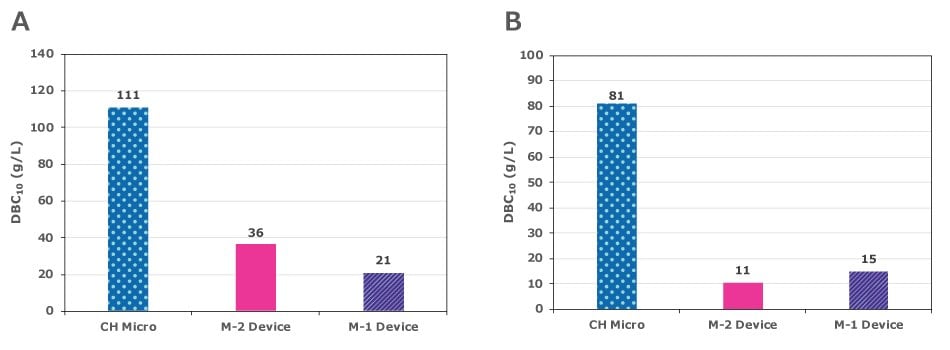
Figure 2.Comparison of DBC of Natrix® CH Micro and conventional membrane adsorbers (M-1, M-2).
Improved Aggregate and HCP Removal
The ability of Natrix® CH and two conventional membrane adsorbers to remove aggregates and HCP impurities from two mAb feed streams was evaluated. Flow-through fractions were collected at different loadings and analyzed for impurities.
Figures 3A and 3B show the feed and flow-through aggregate content for mAb-1 and mAb-2 using either the Natrix® CH or conventional membrane adsorbers. Both feeds were 3 g/L in 50 mM acetate buffer, pH 5.0, 9 mS/cm. At 1,000 g/L loading, flow-through fractions in the feed stream processed using the Natrix® CH device contained less than 2% aggregates as compared to 3-4.5% aggregates with conventional membrane adsorbers.
HCP removal is shown in Figures 3C and 3D. Using a Natrix® CH device at 1000 g/L loading, the HCP content was reduced to ~60 and 40 ppm for mAb-1 and mAb-2 respectively, representing a reduction of 0.9 log for both the mAbs. In contrast, HCP removal for conventional membrane adsorbers M-1 and M-2 ranged from 0.35-0.46 log, indicating inferior HCP clearance as compared to the Natrix® CH device.
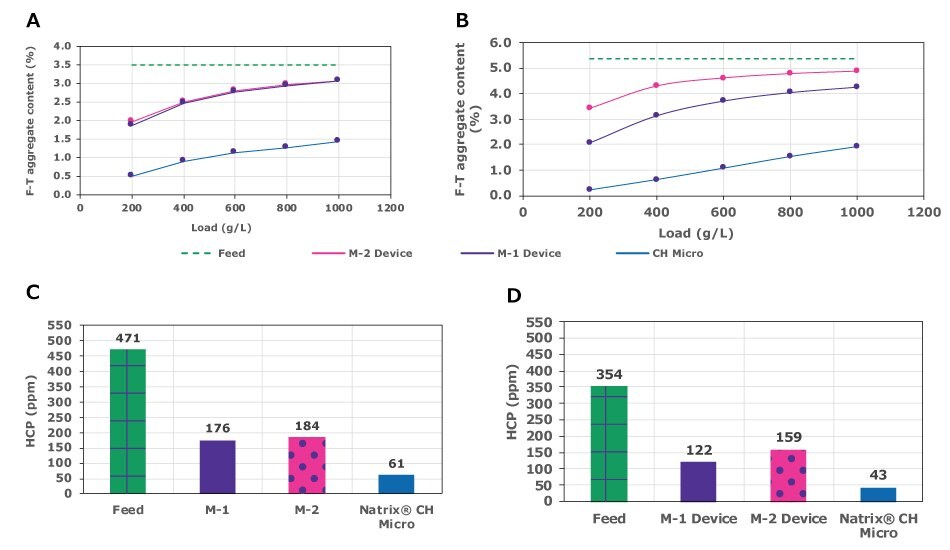
Figure 3.Comparison of aggregate removal (A and B) and HCP removal (C and D) using Natrix® CH Micro or conventional membrane adsorbers (M-1, M-2).
Cycling Performance and Productivity
Impurity removal performance with the Natrix® CH membrane device remains stable over twenty cycles of use. Figure 4 shows the results of a study where a mAb feed stream (19 g/L, 2.2% aggregates, 2000 ppm HCP) was loaded at 1,000 g/L per cycle for 20 consecutive cycles. For all 20 cycles, aggregate content in frontal mode was ~1.7%, HCP content was <100 ppm and product yield was ~95%. While results will depend on specific feed characteristics and run conditions, for these specific conditions, the productivity of the Natrix® CH membrane device was 35x higher than resin.
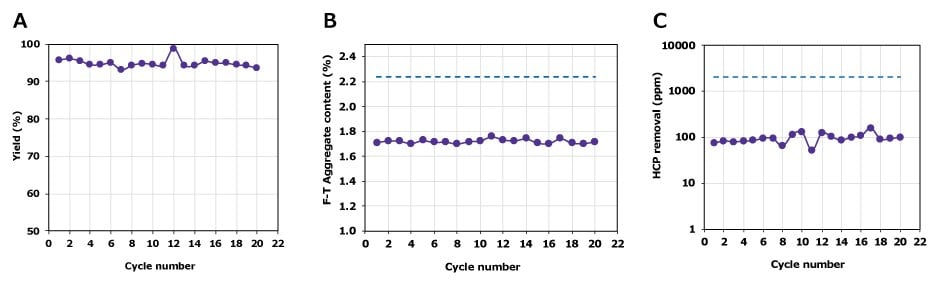
Figure 4.The Natrix® CH device was used for twenty consecutive cycles and maintained a consistent level of aggregate and HCP removal.
The higher capacity of Natrix® CH membrane chromatography devices allows superior removal of mAb aggregates and HCP impurities as compared to two commercially available low-capacity CEX membrane adsorbers. This is achieved by the unique features of the three-dimensional Natrix® CH membrane structure which maximizes binding sites and surface area. The result is high binding at flow rates 40x faster than resin and improved productivity (g/L/hr) up to 35x higher than CEX resin.
This novel CEX chromatography membrane minimizes purification time without sacrificing aggregate or HCP removal and enables intensified mAb processing where speed and flexibility are essential.
Viral Removal Performance
The virus removal performance of the Natrix® CH device during monoclonal antibody (mAb) purification was evaluated with minute virus of mice (MVM) and xenotropic murine leukemia Virus (XMuLV), a parvovirus and model retrovirus, respectively. A post-protein A eluate of mAb (3.7 g/L) in 50 mmol/L acetate buffer, pH 5, 10 mS/cm, was used for this study. This feed was spiked with the appropriate volume of MVM or XMuLV stock to achieve an infectious titer of 1.0×107 TCID50/mL (8.3 Log TCID50 load) and purification was performed in frontal (flow-through) mode at a flow rate of 10 MV/min, with samples collected throughout the run. The virus removal results are shown in Figure 5. For MVM, approximately 3.6 log virus removal was observed at 200 g/L loading with clearance of 3.3 log at 1000 g/L loading. Virus removal for XMuLV was notably better with clearance of 5.7 log at 1000 g/L.
These results demonstrate excellent performance of the Natrix® CH device for virus removal during mAb purification in flow-through mode.
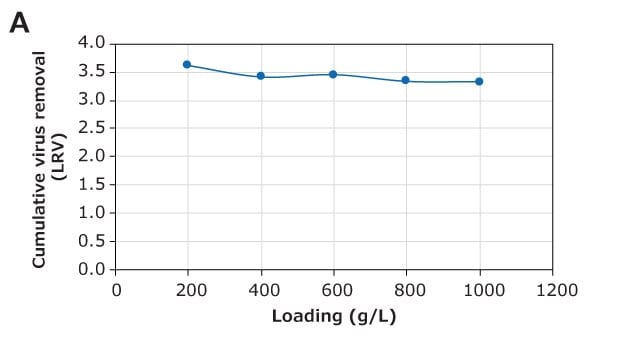
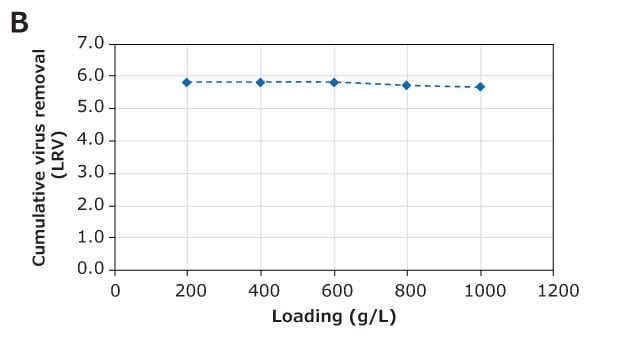
Figure 5. (A) MVM and (B) XMuLV removal during mAb purification using the Natrix® CH device in frontal chromatography mode.
Scale-up Study
The scale-up study for Natrix® CH Micro (1.06 mL), Bench (8.8 mL), Pilot (124 mL), and Process (372 mL) devices was conducted at 10 MV/min. The lysozyme DBC and pressure are shown in Figures 6A and 6B, respectively. The lysozyme DBC for all scales was approximately 110 g/L, indicating excellent scalability. The max delta column pressure (ΔPcol) for all devices was similar (approximately 15 psi).
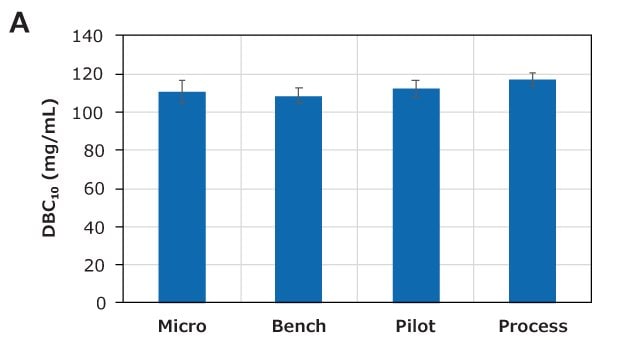
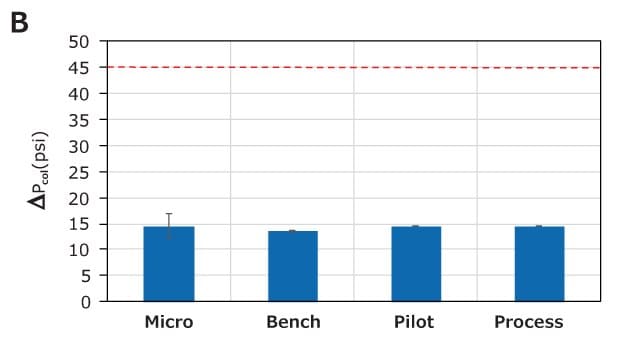
Figure 6. Scale-up performance of Natrix® CH devices. Similar (A) DBC and (B) pressure indicate excellent scale-up. The red dotted line indicates the maximum pressure limit for the devices.
The aggregate and HCP removal performance of the different CH device sizes was also examined in bind-elute mode at a flow rate of 10 MV/min and a 80 g/L loading. The feed contained 3.3% aggregates, which could be reduced to approximately 0.5% for all device sizes, as shown in Figure 7A. Similarly, the different device sizes also had similar host cell protein (HCP) removal performances, as shown in Figure 7B. These purification performances indicate excellent scalability between CH Micro, Bench, Pilot, and Process devices.
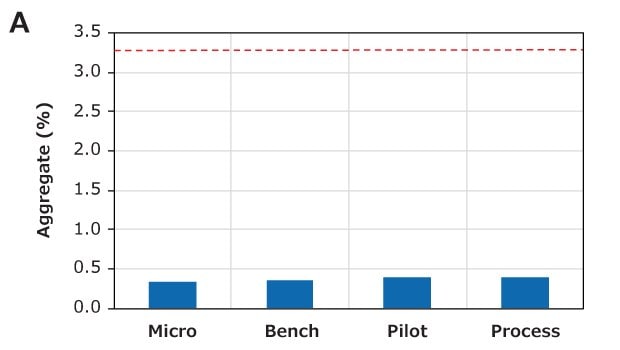
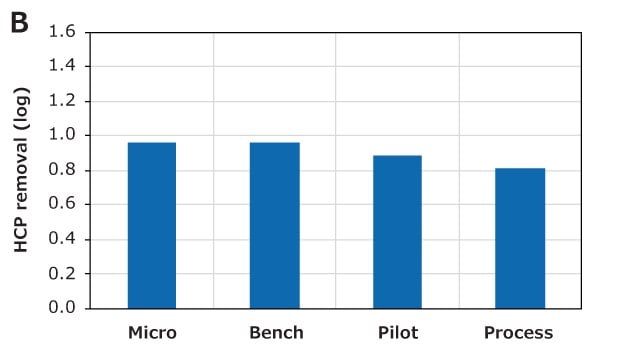
Figure 7. Scale-up performance of Natrix® CH devices. Similar (A) aggregate removal and (B) HCP removal indicate excellent scale-up. The red dotted line indicates the aggregate content in the feed.
Related Products
如要继续阅读,请登录或创建帐户。
暂无帐户?