兽医学和动物健康研究的多重检测
兽医学和动物健康研究具有重大意义,包括保护动物和人类健康及推动科学发展。本文详述了MILLIPLEX®的生物标志物多重免疫检测对于实验室、伴生和农业动物研究的助益。
生物标志物多重免疫检测对于动物研究的助益
动物健康研究涉及多个不同领域,包括兽医学、实验室动物模型以及伴生和农业动物。在这些实验室、伴生和农业动物研究中采用生物标志物多重免疫检测有不少好处。利用多重检测,研究人员既可节省宝贵的时间、成本和样品用量,又可在一次检测中大幅提高数据点数目。
MILLIPLEX®多重检测试剂盒可用于兽医学和动物健康研究
MILLIPLEX®多重检测试剂盒,基于Luminex® xMAP®液相悬浮芯片技术,让科研人员得以开展兽医学、动物健康、动物模型和人类健康研究,了解掌握复杂的生物学体系和过程。目前我们提供的检测试剂盒,囊括伴生、农业、实验室动物和人类的多个研究对象。
MILLIPLEX®试剂盒可用于分析以下种类的动物:
实验室动物
| 伴生动物
| 农业动物
|
这些高度验证的检测试剂盒既能节省时间和样品用量,又能产生最高品质的数据(表1)。
农业动物研究中细胞因子多重分析实例
用于农业动物研究的羊、鸡和牛细胞因子多重检测实例如下。
羊
MILLIPLEX® Ovine Cytokine/Chemokine Panel 1(羊细胞因子/趋化因子多重检测试剂盒)是首个对同一个样品多达14种(可选)羊细胞因子进行多重检测的试剂盒。该panel的检测数据示例如图1和2。

图 1.羊PBMC(BioIVT公司,Hicksville, NY)用LPS或刀豆蛋白A(Con-A)刺激48小时,或不做刺激。根据MILLIPLEX®羊细胞因子/趋化因子Panel 1的使用指南,采集细胞上清并检测(n=3,平均数)。该样品组的IL-8分析物达到标准曲线的最高值。
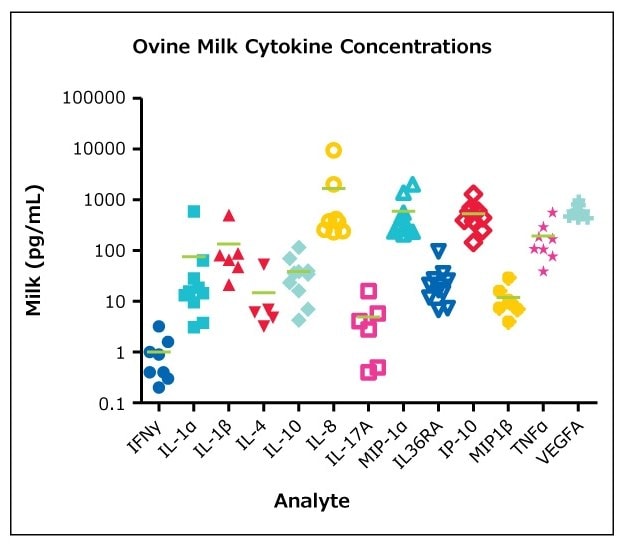
图 2.根据Ovine Cytokine/Chemokine Panel 1的使用指南(n=10, mean),检测MILLIPLEX®羊奶样品(BioIVT公司,Hicksville, NY)。
鸡
MILLIPLEX® Chicken Cytokine/Chemokine Panel 1(鸡细胞因子/趋化因子多重检测试剂盒)是首个对同一个样品多达12种(可选)鸡细胞因子进行多重检测的试剂盒。该panel的检测数据示例如下。(图3)
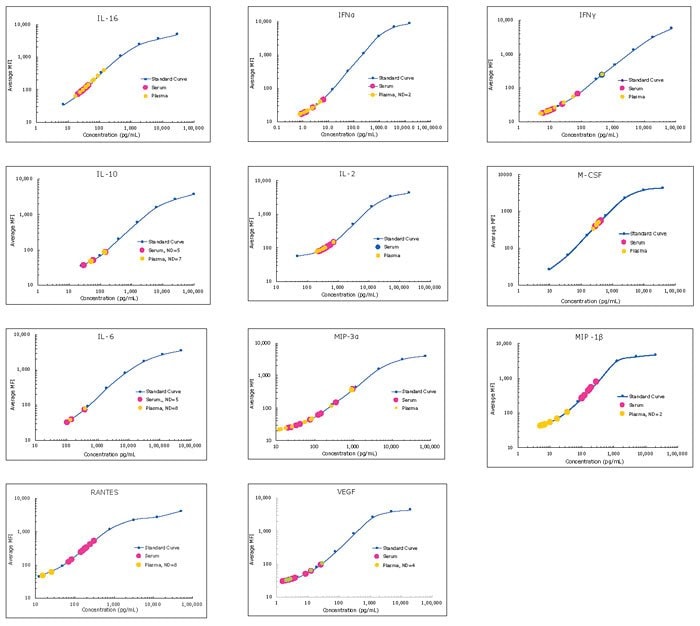
图 3.使用商业来源的正常健康新罕布什尔州鸡的血浆和血清样品(n=8,每份),依照MILLIPLEX® Chicken Cytokine/Chemokine Panel 1的过夜方案(Overnight Protocol)进行检测。“ND=n”(未检测=n)表示在检测中某种分析物未检出的样品数目。这些样品未检出IL-21分析物,但可以预计的是,对某些疾病/炎症状态的检测会显示IL-21值。
牛
MILLIPLEX® Bovine Cytokine/Chemokine Panel 1(牛细胞因子/趋化因子多重检测试剂盒)是首个对同一个样品多达15种牛细胞因子进行多重检测的试剂盒。图4和5显示两种样品类型的分析物数据示例。
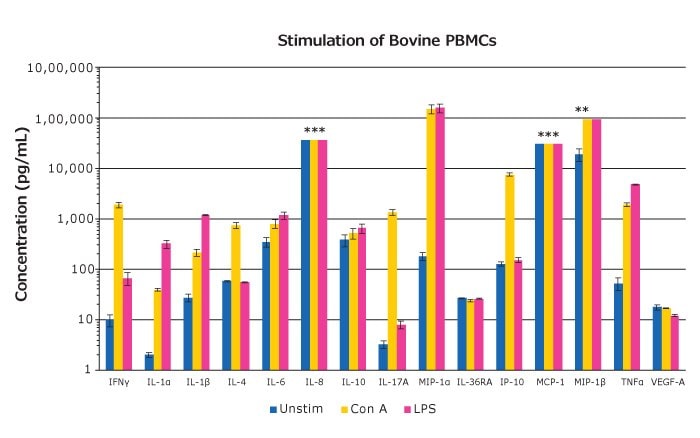
图 4.牛PBMC(BioIVT公司,Hicksville, NY)用LPS或刀豆蛋白A(Con A)处理48小时,采集无细胞样品并用MILLIPLEX® Bovine Cytokine/Chemokine Panel 1进行检测 (n=3 mean ± SEM)。*表示这些样品组的某分析物达到标准曲线的最高值。
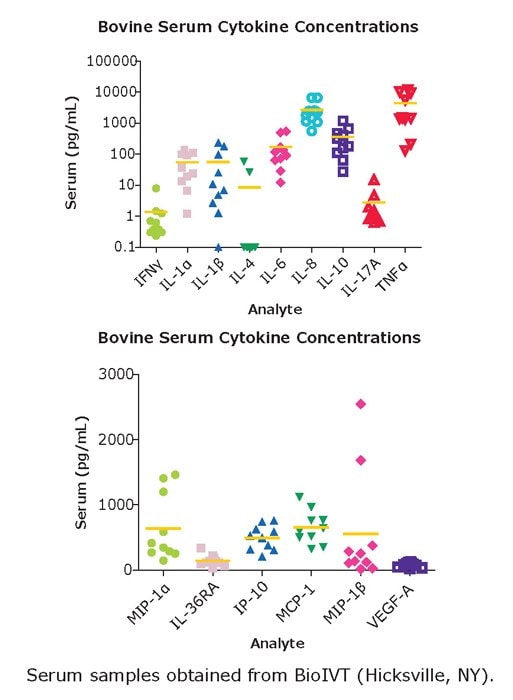
图 5.血清样品来自BioIVT公司(Hicksville, NY)。根据MILLIPLEX® Bovine Cytokine/Chemokine Panel 1的实验方案检测样品。
伴生动物研究中垂体激素多重分析实例
在伴生动物研究中实行的牛垂体激素多重检测实例如下。
使用MILLIPLEX® Canine Pituitary Expanded Panel对血清、血浆和细胞/组织培养样品中多达6种牛垂体激素分析物进行定量检测。分析物数据示例如图6。
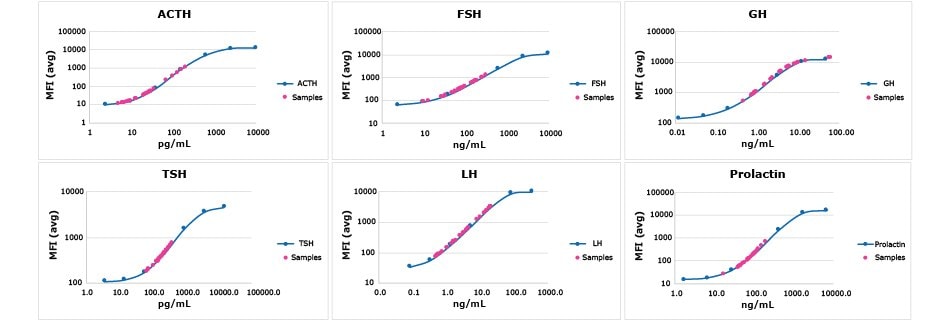
图 6.根据MILLIPLEX® Canine Pituitary Expanded Panel的实验方案,对商业来源的正常牛血清(n=22)和血浆(n=27)样品进行检测。洋红色圆圈代表样品落在所示分析物的标准曲线上。
重点客户访谈
采用大鼠细胞因子检测试剂盒进行Zika研究的客户访谈
观看Mukesh Kumar博士的访谈视频,了解他使用MILLIPLEX® Rat Cytokine/Chemokine Magnetic Bead Panel进行Zika病毒研究的相关内容,并阅读他在《Virology Journal》刊物上发表的研究文章。
采用牛细胞因子/趋化因子多重检测试剂盒进行农业研究的问答访谈
阅读我们与田纳西大学农业研究所动物科学部门反刍动物繁殖科副教授Kyle McLean博士的访谈实录,了解MILLIPLEX®检测试剂盒如何助力其农业动物研究。
请简单介绍一下您目前的研究。
目前我主要研究反刍动物繁殖,特别是子宫环境、胎盘发育和妊娠早期的胎儿编程。
牛在您研究中的作用?
牛是我的研究重点。
为什么选择牛?
因为牛的经济意义和生物医学潜力,它在现在和未来都是我的研究重心。
MILLIPLEX® Bovine Cytokine/Chemokine Panel 1对于您的研究和/或实验流程有何帮助?
这款Panel让我能用更少的样品定量分析更多的细胞因子,而且消耗的时间最短。还能用来建立子宫环境的细胞因子谱。
您实验室还负责哪些重要工作?
我们也在建立子宫环境的氨基酸谱,研究营养对公牛精浆成分的影响。
如果您能解决某个研究难题,您希望是?
了解妊娠建立的潜在机制,确定妊娠期间母亲和胚胎的营养需求。
对于初涉您研究领域的科学家,有什么建议?
不要畏惧挑战难题,多做创造性思考
小鼠
大鼠
非人灵长类
犬
猫
牛
马
猪
羊
鸡
如果您没有找到需要的检测产品,我们也可提供定制检测服务,根据您研究所需的多重检测方式,开发适合的检测产品。
仅供研究使用,不可用于诊断。
重要发表文章*
可查看MILLIPLEX®多重检测在兽医学和动物健康研究中的应用。也可探索这些试剂盒如何用于动物研究模型,比如这篇高灵敏度小鼠细胞因子panel的评价是关于小鼠模型的。
伴生动物
犬
猫
农业动物
牛
马
猪
羊
仅供研究使用,不可用于诊断。
如要继续阅读,请登录或创建帐户。
暂无帐户?