Online SPE/LC-MS Method for the Rapid Analysis of Thyroid Hormones in Plasma
Using Ascentis® Express Fused-Core® Columns for Trapping and Separation
The power of bonded phase selectivity in both sample prep and separation is demonstrated here. The Ascentis Express RP-Amide was shown to be a better trapping phase than conventional C8, and the Ascentis Express Phenyl-Hexyl phase provided baseline resolution of the closely-related compounds. The technique uses standard hardware and is a viable approach to solve other clinical and bioanalytical challenges.
Introduction
Thyroid hormones play critical roles in the regulation of biological processes such as growth, metabolism, protein synthesis, and brain development. Specifically, thyroid hormones 3,3´,5,5´-tetraiodo- L-thyronine (thyroxine or T4) and 3,3´,5-triiodo-L-thyronine (triiodothyronine or T3) are essential for the development and maintenance of normal physiological functions. For a clinical laboratory, measurements of total T4 and total T3, along with estimates of free T4 (FT4) and free T3 (FT3) are important for the diagnosis and monitoring of thyroid diseases. The physiological role and need for testing of 3,3´,5´-triiodo-L-thyronine (reverse triiodothyronine or rT3) is debated in clinical research circles.
Analytical Challenges of Thyroid Hormone Measurement
Thyroid hormones are typically measured by an immunoassay technique that is subject to assay interference and easily perturbed by changes in protein levels that alter the free hormone availability1. Liquid chromatography with mass spectrometric detection (LCMS) has been reported to offer superior specificity and speed over immunoassays for the determination of thyroid hormones in biological matrices, such as serum, plasma, and tissue. Nevertheless, the reported sample preparation procedures, typically by liquid-liquid extraction followed by solid phase extraction (SPE), involve multiple time-consuming steps, and are less compatible with automation2,3.
Study Goal and Overview of Approach
The present work exploited online SPE with LC-MS for the rapid determination of T4, T3, and rT3 from plasma. The structures of these compounds appear in Figure 1. A 100 µL aliquot of rabbit plasma was spiked with T4, T3, and rT3. Protein was precipitated by addition of 25 µL ZnCl2, and 200 µL methanol, vortex agitation for 1 minute, and centrifugation at 9,000 g for 3 minutes. The resulting supernatant was collected and injected into the online SPE/LC-MS system described below. The LC-MS conditions are described in Table 1.
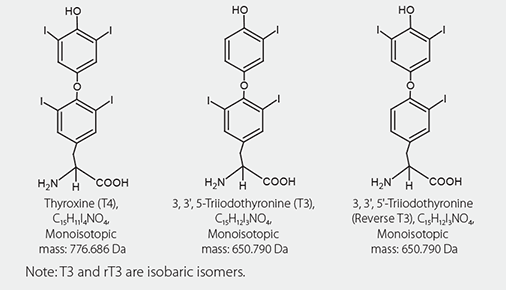
Figure 1.Chemical Structures of Thyroid Hormone Analytes
Online SPE/LC-MS Program
The online SPE/LC-MS system shown in Figure 2 comprises a trapping column, a switching valve and an LC-MS instrument. The program consists of three distinct steps outlined in Table 2. The valve switches between position 1 for loading/washing (Step 1) and equilibration (Step 3), and position 2 for LC-MS analysis (Step 2). In Step 1, samples are loaded onto the trap and washed with mobile phase containing low percent organic and high flow rate to remove salts and other interferences which are directed to the waste. In Step 2, the analytes are eluted from the trap, separated and detected by the LC-MS at optimum flow for both chromatographic separation and MS signals. In Step 3, the system returns back to valve position 1 for re-equilibration under the sample loading/washing conditions.
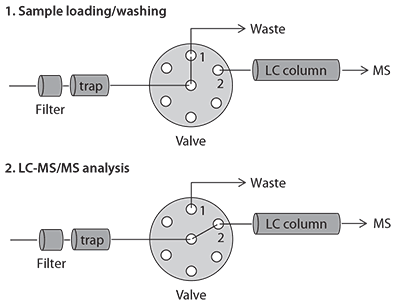
Figure 2.Configuration of the LC-MS System and Valve Positions for Online SPE/LC-MS
Choosing the Trapping Column
The choice of the right trapping column is critical for successful online SPE/LC-MS method development as it affects both trapping efficiency (e.g., the recovery of the analytes) and the downstream LC separation. As a rule of thumb, the trapping column should be less retentive than the analytical column to avoid possible peak broadening. Two guard cartridges (traps), Ascentis Express Fused- Core RP-Amide and C8, of the same dimensions were evaluated. As can be seen in Figure 3, both trapping columns led to comparable sharp peak shape (high separation efficiency). However, the RP-Amide delivered higher peak responses than the C8 trap under the trapping conditions with 5% methanol (top panel) as the washing solvent. Further increase of the methanol content to 20% in the washing solvent (Table 3) resulted in a 50% signal decrease on the C8 traps, but almost no change in signals with the RP-Amide traps (Figure 3, bottom panel). These results indicate better washing can be achieved with minimal sample loss with RP-Amide traps.
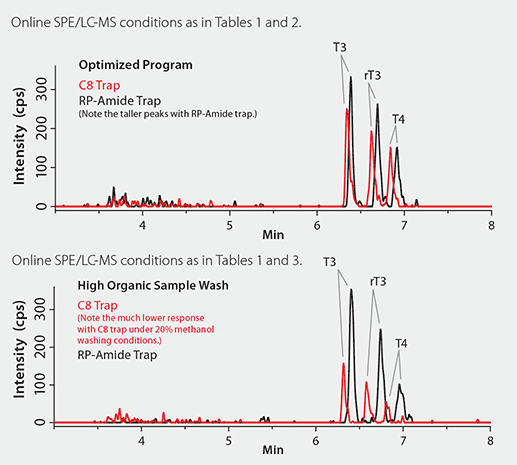
Figure 3. Comparison of the LC-MS Chromatograms with Online Trapping with C8 and RP-Amide Guard Cartridges
Summary
An online SPE/LC-MS method was developed and described here for the determination of the thyroid hormones T4, T3, and rT3 from plasma. The method uses an Ascentis Express RP-Amide cartridge to trap the analytes, and an Ascentis Express Phenyl-Hexyl column to resolve them. Compared to the commonly used C8 trap, the RP-Amide trap gave higher analyte signals and was compatible with 20% methanol washing. Another advantage of the RP-Amide traps is their compatibility with 100% aqueous mobile phases and samples. This is due to the hydrophilic nature of the amide-embedded phase, which makes it very useful for polar analytes.
Legal Information
Ascentis is a registered trademark of Sigma-Aldrich Co. LLC
Fused-Core is a registered trademark of Advanced Materials Technology, Inc.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?