Protocol Guide: Microglia Monoculture
What is a Microglia?
A major immune cell within the central nervous system (CNS), microglia are responsible for eliminating dead neurons, microbes, protein aggregates, and other particulates like degenerating synapses and myelin debris that are in the CNS. They also help to maintain neuronal networks, aid in injury repair, and mediate neuroinflammation. Microglia are implicated in neurodegeneration and aging, and are used to study autism, epilepsy, Alzheimer’s disease, and Huntington’s disease.
Our collection of microglia, provided through our partnership with BrainXell, are generated via a hematopoietic progenitor lineage. They are fully differentiated and express U1 and IBA1 at DIV 7. Here we provide a protocol for seeding and culturing microglia.
Section Overview
Microglia Culture Materials
Immediately transfer the neuron cryovials to a liquid or vape nitrogen storage system.
- BrainXell Human Microglia (BX-0900-30-1M or BX-0900-32-1M)
- DMEM/F12 Medium, +L-glutamine, +HEPES (D0697)
- B-27 Supplement (Thermo Fisher Scientific #17504044)
- N-2 Supplement (Thermo Fisher Scientific #17502048)
- GlutaMAX Supplement (Thermo Fisher Scientific #35050061)
- MEM NEAA (Thermo Fisher Scientific #11150050)
- Chemically Defined Lipid Concentrate (Thermo Fisher Scientific #11905031)
- Cultrex (R&D Systems #3432-001-01)
- Ascorbic Acid (A8960)
- M-CSF (SRP3110)
- IL-34 (SRP3287)
- TGF-β1 (SRP0300)
- PDL-coated 96-Well Plate (M0812, LPDL001)
Microglia Culture Protocol
Day 0: Seeding Preparation
Seed 2.2-3.3 million live cells for a 96-well plate. Additional cells can be used depending on experimental design - refer to the Certificate of Analysis for viability and seeding information.
- Combine all components listed below in the cell culture hood or biological safety cabinet to make the Microglia Basal Medium. Allow to equilibrate at room temperature for 15 minutes, but do not place medium in a 37°C water bath. This medium stock can be stored at 4°C for up to 2 weeks.
- The addition of growth factors is optional – recommended final concentrations are: 2µg/ml TGF-β1, 100µg/ml M-CSF, and 100µg/ml IL-34.
*To make the Ascorbic Acid solution, click here.
- Add 495µl of cold DMEM/F12 medium into a 55µl aliquot of frozen Cultrex to prepare the pre-diluted Cultrex solution. Mix to dissolve and store at 4°C until experimentally necessary.
- Prepare a 50ml conical tube with 3ml of Basal Medium and a separate microcentrifuge tube with 25µl of Trypan Blue solution for cell counting.
Day 0: Seeding the Microglia
- Remove microglia cryovial from liquid nitrogen storage and place in a 37°C water bath to thaw. To minimize contamination, do not submerge the cap.
- Remove the vial from the water bath when the last of the ice melts (~75-90 seconds) and disinfect the vial with 70% ethanol. Transfer the microglia vial to the cell culture hood.
- Using a P1000 pipette, transfer 500µl of the Microglia Basal Medium to the cell vial from the prepared 50ml conical tube at a rate of 2-3 drops/sec; this should take about 10 seconds.
- Transfer the 1ml Microglia cryovial contents to the 50ml conical tube.
- Centrifuge the cell solution at 200xg for 5 minutes.
- Aspirate the supernatant from the pellet and resuspend the pellet in 950µl fresh Microglia Basal Medium by gently pipetting with a P1000 pipette.
- Add Microglia Basal Medium to the existing 1ml in the conical tube to resuspend the cells to a concentration of 1.0 x 106 live cells/ml based on the CoA value.
- For example, 2.3 million viable cells/vial is diluted to 2.3 total volume.
- For example, 2.3 million viable cells/vial is diluted to 2.3 total volume.
- Count the cells using the prepared Trypan Blue solution. Evenly mix the Microglia cell solution by pipetting up and down 3-5 times and transfer 25µl of the cell suspension to the prepared microcentrifuge tube from Step 2. Pipette up and down to mix. Determine the live cell concentration and viability by counting the number of viable and dead cells using a hemocytometer.
- Calculate the volume needed to make the Microglia seeding suspension; the typical seeding density is 20,000-30,000 viable cells/100µl/well for a 96-well plate or ~62,500-93,750 viable cells/cm2. The recommended seeding density may vary, so refer to the CoA for lot-specific seeding information. Based on the Trypan Blue cell count, dilute cells to the desired seeding concentration.
Example dilution calculation:
- Mix the calculated volumes of Microglia Basal Medium with cell suspension in a new 50ml conical tube to make the 11ml final volume Microglia seeding suspension solution.
- Make the Microglia Seeding Medium:
*Cultrex should be added to the final seeding suspension immediately before seeding the plate.
- Completely mix seeding medium and transfer 100µl/well to a PDL-coated 96-well plate.
NOTE: Do not agitate the plate during the seeding process – this can lead to uneven cell attachment.
- Allow cells to settle to the bottom of the wells by resting the plate for 10 minutes after seeding. Transfer the plate gently to a humidified incubator set at 37°C with 5% CO2.
This seeding protocol should not exceed one hour, otherwise the post-thaw health and viability of the cells may be negatively impacted.
Day 4: Microglia Medium Addition
- Prepare the Day 4 Microglia Medium fresh 96 hours after seeding:
- Add 100µl/well of Day 4 Microglia Medium to the entire plate for a final volume of 200µl/well.
Day 7 and Onward Microglia Medium Changes
- Using the Microglia Basal Medium, change half of the media weekly (i.e. on Day 7, 14, 21, etc.). Aspirate 100µl/well of the medium in the well and slowly add 100µl/well of Microglia Basal Medium to the entire plate.
- The addition of BrainFast SK at low concentrations (0.1-0.5X) may be helpful for long-term cultures.
The microglia mature rapidly and can be maintained in culture under the above conditions for at least 3 weeks after seeding.
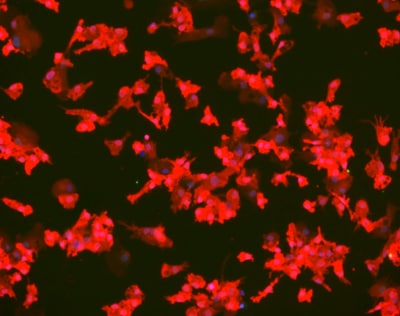
Figure 1.Immunofluorescent image of microglia
Related Materials*
*These products are available in only the US and UK.

如要继续阅读,请登录或创建帐户。
暂无帐户?