Comparison of Ascentis® Express C18 Column Stationary Phases for Analysis of Basic Analytes
Abstract
This study evaluates the performance of various Ascentis® Express C18 column phases for the analysis of basic analytes under two acidic mobile phase conditions, highlighting the superior peak shapes achieved with the PCS-C18 column, which enhances method efficiency and applicability in LC-MS.
Section Overview
Introduction
In this study, we assess the performance of different Ascentis® Express C18 column phases in the analysis of four basic analytes (nebivolol hydrochloride, dextromethorphan hydrobromide, sitagliptin phosphate, and hydroxyamphetamine hydrobromide) using low ionic strength mobile phases. Working with basic compounds is challenging due to their ability to undergo strong secondary interactions with the base silica of C18 columns, thereby producing unsymmetrical peak shapes and compromising the method’s efficiency.
The new Ascentis® Express PCS-C18 column features a positively charged surface (PCS) stationary phase, enabling the retention and elution of basic compounds with significantly improved peak shape under acidic conditions. In this paper, the separation of four basic analytes (Figure 1) was studied on four different Ascentis® Express phases with C18 functionality using weak acidic mobile phase buffers: the well-established Ascentis® Express C18 stationary phase, as well as the more recently introduced columns Ascentis® Express AQ-C18, Ascentis® Express ES-C18, and Ascentis® Express PCS-C18.
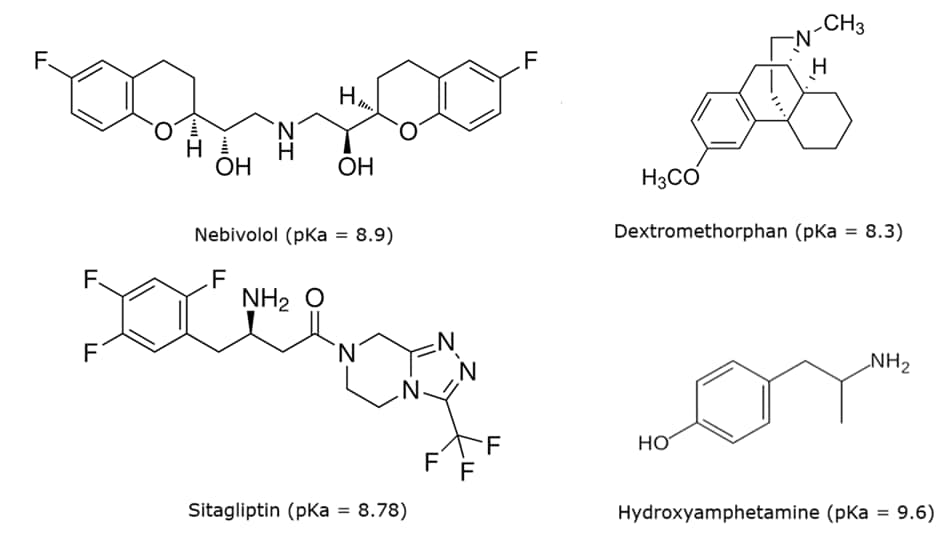
Figure 1.Chemical structures of the basic analytes and their pKa values.1-4
Experimental
Reagent Preparation
The analysis of the basic analytes was separated into two sets using two different acidic mobile phase compositions. Preparation of the mobile phases for the two sets was as follows:
Mobile Phase Set I
- Mobile phase A: Weigh and dissolve 0.630 g ammonium formate in 1000 mL water. Adjust the pH to 3.0 with formic acid to obtain an aqueous solution of 10 mmol/L ammonium formate, pH 3.0.
- Mobile phase B: Weigh and dissolve 0.630 g ammonium formate in 200 mL water (50 mmol/L), and mix with 800 mL acetonitrile to obtain a mixture of acetonitrile and 50 mmol/L ammonium formate aqueous solution (80:20, v:v).
Mobile Phase Set II
- Mobile phase A: Add 1 mL formic acid to 1000 mL water and mix well to obtain a solution of 0.1% formic acid in water.
- Mobile phase B: Add 1 mL formic acid to 1000 mL acetonitrile and mix well to obtain a solution of 0.1% formic acid in acetonitrile.
Standard Preparation
Standard mix solution: Weigh 20 mg each of nebivolol hydrochloride, dextromethorphan hydrobromide, sitagliptin phosphate, and hydroxyamphetamine hydrobromide into a 50 mL volumetric flask. Add 25 mL methanol and sonicate for 5 minutes. Fill to volume with water and mix well. The resulting solution contains 400 µg/mL of nebivolol hydrochloride, dextromethorphan hydrobromide, sitagliptin phosphate, and hydroxyamphetamine hydrobromide.
HPLC-UV Method
The standard solution was analyzed by HPLC-UV using the two sets of mobile phase compositions (Tables 1 & 2).
Results
The performance of the four Ascentis® Express C18 columns for the analysis of basic analytes using set I acidic mobile phase composition is demonstrated in Figure 2, and using set II acidic mobile phase composition is demonstrated in Figure 4. Tables 3 & 4 summarize the obtained chromatographic results, including the determined USP symmetry factors (also known as asymmetry or tailing factor, TUSP). Figures 3 & 5 provide a visual comparison of the achieved tailing factors for sets I & II demonstrating the PCS-C18 to provide the most symmetrical peaks and meet the common acceptance window of 0.8-1.8 (USP chapter <621>) for all compounds under the chosen conditions. This is particularly evident for the mobile phase set II (0.1% formic acid).
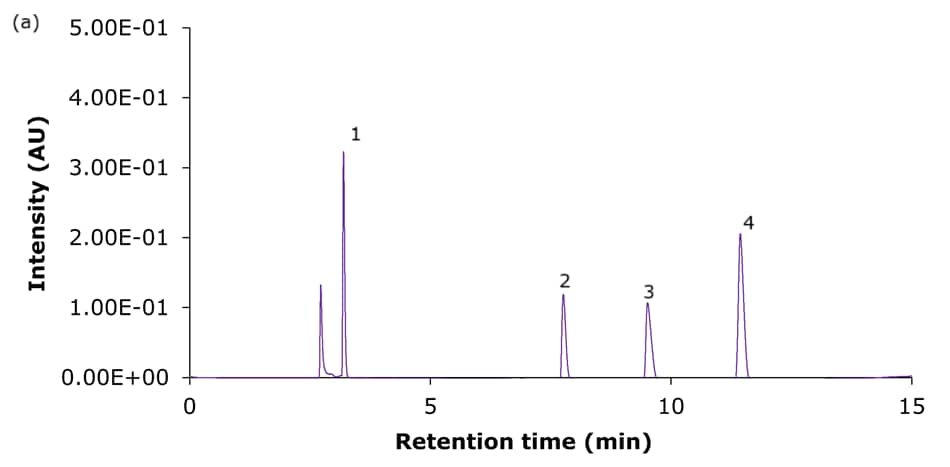

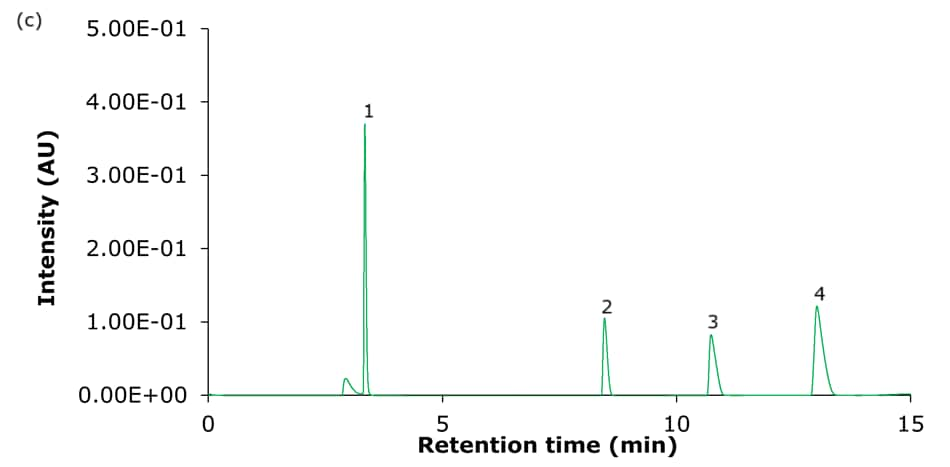
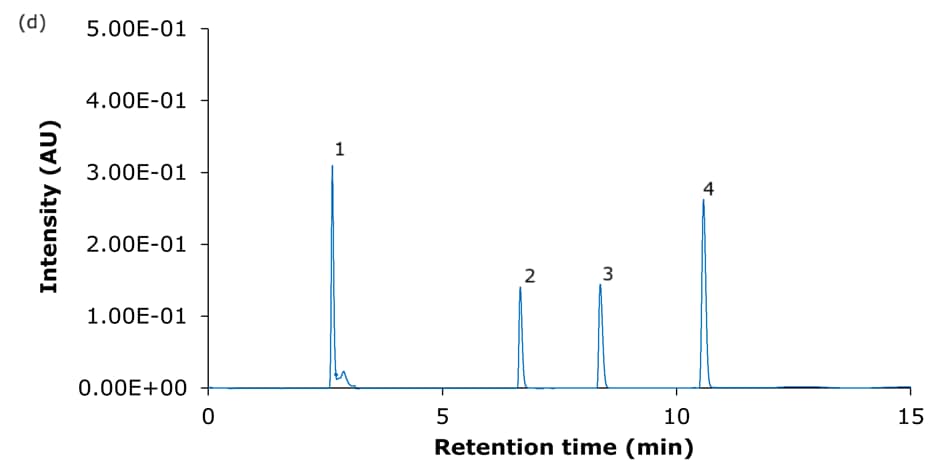
Figure 2. Representative chromatograms of the analysis of the four basic analytes (1) hydroxyamphetamine, (2) sitagliptin, (3) dextromethorphan, and (4) nebivolol on Ascentis® Express 90 Å phases with C18 functionality - (a) C18, (b) AQ-C18, (c) ES-C18, and (d) PCS-C18 - 15 cm x 4.6 mm, 2.7 μm columns using acidic mobile phase set I. [Table 1, 10 mmol/L ammonium formate in water, pH 3.0/acetonitrile:50 mmol/L ammonium formate in water (80:20 v:v)]
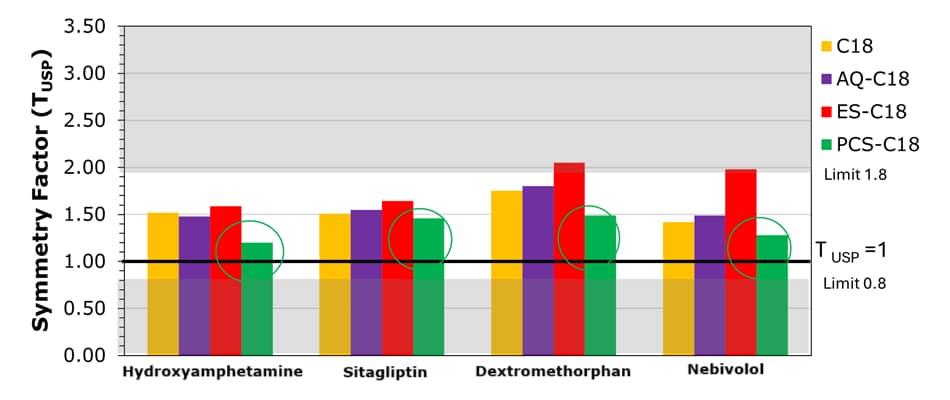
Figure 3.Tailing factors for mobile phase set I demonstrate that the PCS-C18 provides symmetry factors (TUSP) for all compounds within the range limits of 0.8-1.8 (USP chapter <621>), with values closest to the ideal TUSP = 1.
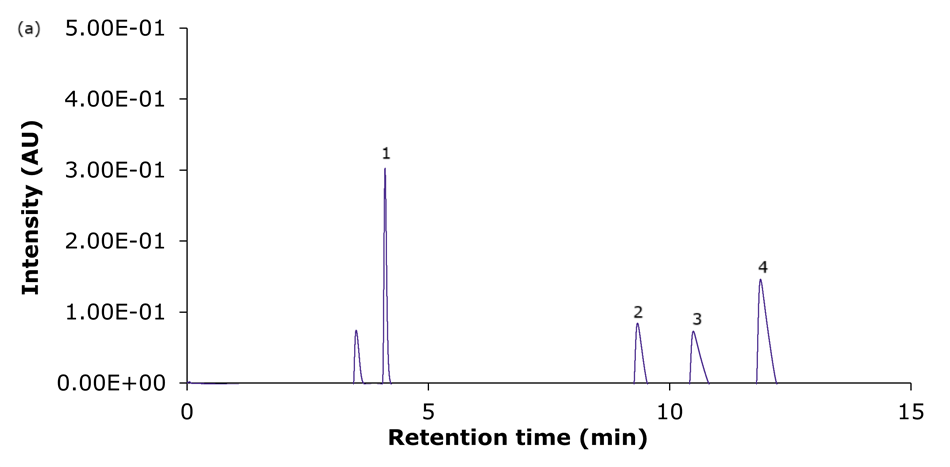
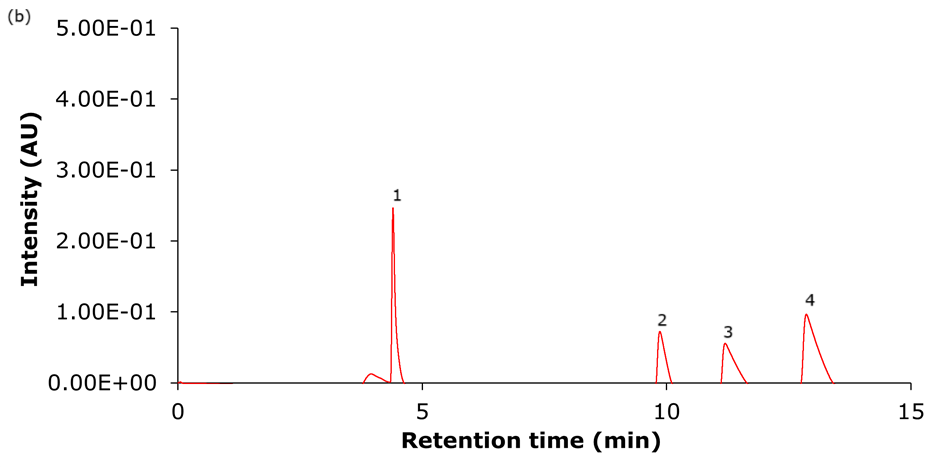
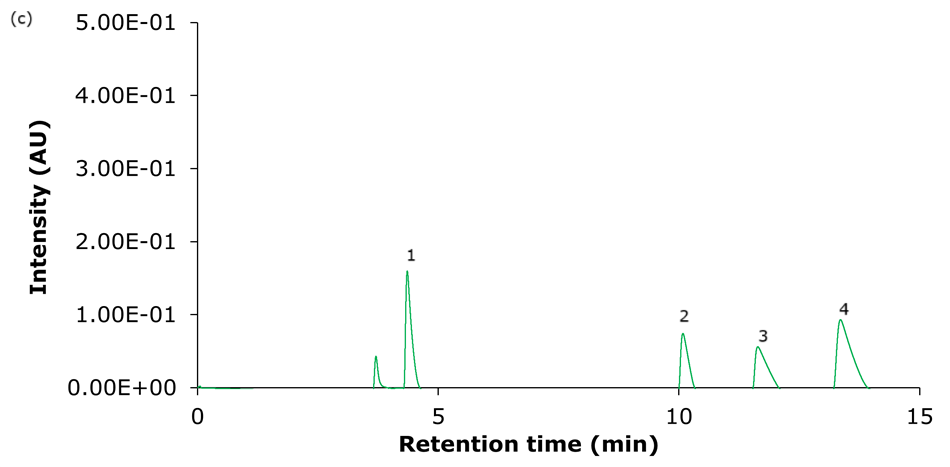
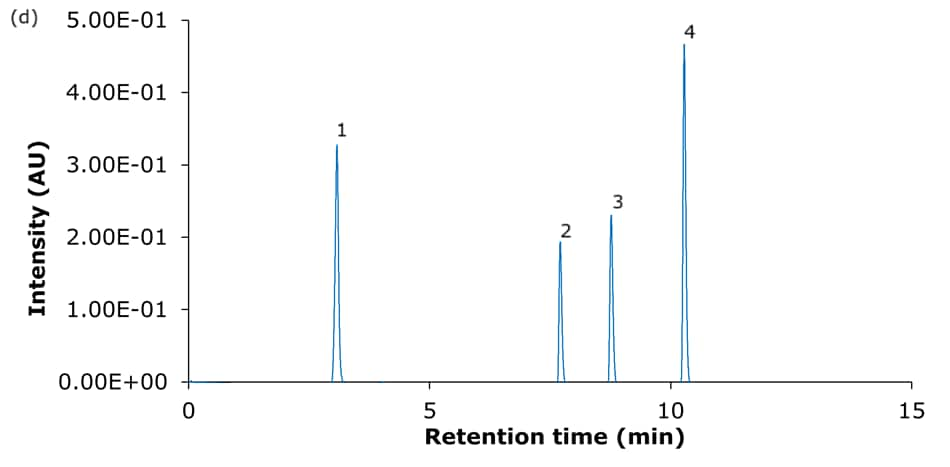
Figure 4. Representative chromatograms of the analysis of the four basic analytes (1) hydroxyamphetamine, (2) sitagliptin, (3) dextromethorphan, and (4) nebivolol on Ascentis® Express 90 Å phases with C18 functionality - (a) C18, (b) AQ-C18, (c) ES-C18, and (d) PCS-C18 - 15 cm x 4.6 mm, 2.7 μm columns using acidic mobile phase set II (Table 2: 0.1% formic acid in water/0.1% formic acid in acetonitrile).

Figure 5.Tailing factors for mobile phase set II indicate that the PCS-C18 provides symmetry factors (TUSP) for all compounds within the range limits of 0.8-1.8 (USP chapter <621>), with values closest to the ideal TUSP = 1.
Conclusion
In comparison to the other Ascentis® Express phases with C18 functionality, the Ascentis® Express 90 Å PCS-C18 provided better peak shapes for the investigated basic analytes when used with low ionic strength mobile phases, which would also benefit LC-MS applications. The significant role and influence of silanol activity on the chromatographic behavior of basic analytes was underlined by the comparative results of the PCS-C18 to the other C18 phases:
- The dramatic improvements in tailing factors with the PCS-C18, in particular when using 0.1% formic acid as a mobile phase modifier, and
- The reduced retention of the analytes on the PCS-C18 phase.
The narrower optimized peak shape results in increased efficiency and peak capacity, allowing for the development of faster and more reliable applications.
This makes the Ascentis® Express 90 Å PCS-C18 column an ideal option for separating basic analytes under acidic conditions and analyzing complex basic mixtures.
Related Products
Discover our selection of the Ascentis® Express 90 Å PCS-C18 (2.7 µm) columns.
HPLC Columns, Reagents, Solvents , & Reference Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?