Sterile Filter Selection for Cell Culture Media Preparation
Contaminants in cell culture media and buffers such as bacteria, fungi, and viruses can proliferate in cell culture environments, leading to inaccurate results. Filtration effectively reduces these microorganisms, ensuring a sterile environment for cell growth. Complete media, base media, serum, buffers, supplements, antibiotics, growth factors, and cell dissociation solutions require filtration.
Read more about:
Importance of Filtration in Cell Culture Media Preparation
- Sterility: Contaminants such as bacteria, fungi, and viruses can proliferate in cell culture environments, leading to skewed results or experimental failure. Filtration effectively reduces these microorganisms, ensuring a sterile environment for cell growth.
- Particulate Matter: Even small particles can adversely affect cell behavior and viability. Filtration eliminates particulates, providing a clear and consistent medium that supports optimal cell function.
- Consistency and Reproducibility: Variability in cell culture conditions can lead to inconsistent results. By filtering media and reagents, researchers can ensure that each batch used in experiments is uniform, facilitating reproducibility and reliability in outcomes.
- Cell Health: Contaminants can induce stress responses in cells, affecting their growth and function. Filtered solutions promote a healthier environment, allowing for more accurate assessments in research and development.
- Regulatory Compliance: For products intended for therapeutic applications, stringent regulatory standards require that all components be sterile. Filtration is a key step in meeting these safety and quality requirements.
General Considerations for Sterile Filter Selection
- Filter Material: Durapore® PVDF and Millipore Express® PLUS PES membrane filters are preferred for their low protein binding characteristics, which are crucial for maintaining the integrity of sensitive solutions. MF-Millipore® mixed cellulose ester (MCE) membranes can also be used for buffer preparations without additives.
- Filter Rating: Sterility filters have 0.2 or 0.22 µm pore ratings, filters for clarification have 0.45 µm pore ratings, and filters for viral or mycoplasma reduction have 0.1 µm pore ratings. Micron-rated nets can also be used for cell straining (10 - 100 µm).
- Sterility: Ensure that filters are certified sterile and suitable for the intended application.
- Filtration Speed: Larger volumes may require a vacuum system to expedite the filtration process, as syringe filters can become clogged with larger volumes or viscous solutions.
- Volume: Refer to device capacity to determine applicable volumes for solutions to be filtered. We offer sterile filtration products for volumes up to 20 L.
- Pre-Filtering: For particularly viscous solutions or those with particulate matter, consider using a pre-filter (e.g., 1.0 µm) before the final 0.22 µm filtration to prolong filter life.
Sterile Filter Selection by Cell Culture Media Component and Application
Cell culture media serve as the relevant “energy source in cell culture that constitutes balance of amino acids, glucose, inorganic salts and serum as a foundation of hormones, growth factors and attachment factors.”1 Media formulations cannot all be sterilized in the same manner. Certain components within the media may degrade or precipitate following heat-steam sterilization, necessitating the exploration of alternative sterilization methods. Vacuum or positive-pressure filtration is frequently employed for liquids that are incompatible with steam sterilization.
The selection of an appropriate filter for a given cell medium or reagent begins with pore size rating. Sterilizing grade 0.22 µm rated filters must pass the American Society for Testing and Materials (ASTM®) method F838-05 test, ensuring that the filter can retain at least 1 x 107 colony forming units (CFU) per cm2 of a challenge bacterium. Filters such as the 0.22 µm Durapore® PVDF, Millipore Express® PLUS PES, and MF-Millipore™ MCE membranes have such ratings. For water and buffers, or when protein or analyte binding is not a concern, use 0.22 µm PES for fast flow or MCE filters for thermal stability. Use 0.22 µm Durapore® PVDF for low protein or analyte binding. Ratings of 0.45 µm indicate clarification uses, and those with 0.1 µm ratings can reduce mycoplasma concentrations.
Convenient and sterile filter formats can be selected based on volume requirements and available equipment. For applications involving syringe or positive pressure systems such as pressure vessels or peristaltic pumps, the Millex® and Sterivex™ filter families are suitable, accommodating volumes from 1 to 200 mL and less than 2 L, respectively. Vacuum-based processing can be achieved using the Steriflip® device (50 mL) or the Stericup® and Steritop® vacuum filtration systems, which manage volumes ranging from 125 mL to over 2 L. For even larger volumes, Stericap® PLUS vacuum filter units are capable of processing 5 to 10 L of typical cell culture media reconstituted from powder, such as DMEM supplemented with 10% serum. For maximum laboratory-scale filtration option, Steripak™ filters can be used for pressure-driven applications of 10 L and 20 L volumes.
Refer to Table 1 for more information on filter selection.
Cell Culture Filtration Applications
Filter sterilization of cytokine-supplemented media for T cell cultures
T cells require contamination-free cell culture media that contain cytokines, serum, and supplements optimized for cell viability and expansion. The right filter membrane will remove particulates and contaminants while preserving the integrity of key heat-labile growth factors and supplements required for T cell function. We examined the impact of repeated filtration of cell culture media containing vital T cell factors and other media components using 0.22 µm Stericup® sterile filtration devices. The impact of filtered media on T cell growth, morphology, marker expression, and cytokine secretion was thoroughly evaluated. The formulated T cell media consisted of Hematopoietic Cell Medium (Lonza Bioscience #BEBP02-054Q), 2% Human Serum, and 50 IU/mL IL-2 prepared in 100 mM acetic acid with 1 mg/mL BSA.
Filtration of the sterile culture media using Stericup® PVDF and PES devices demonstrated no adverse effects on T cell health, phenotype, viability, cell concentration, or cell diameter over a six-day period (see Figure 1 for sample data). Additionally, no contamination of the media was observed throughout the experiment. The use of Stericup® devices for filtering cell culture media ensures the viability and seamless expansion of T cells, thereby supporting the production of T cell and CAR-T cell therapies.
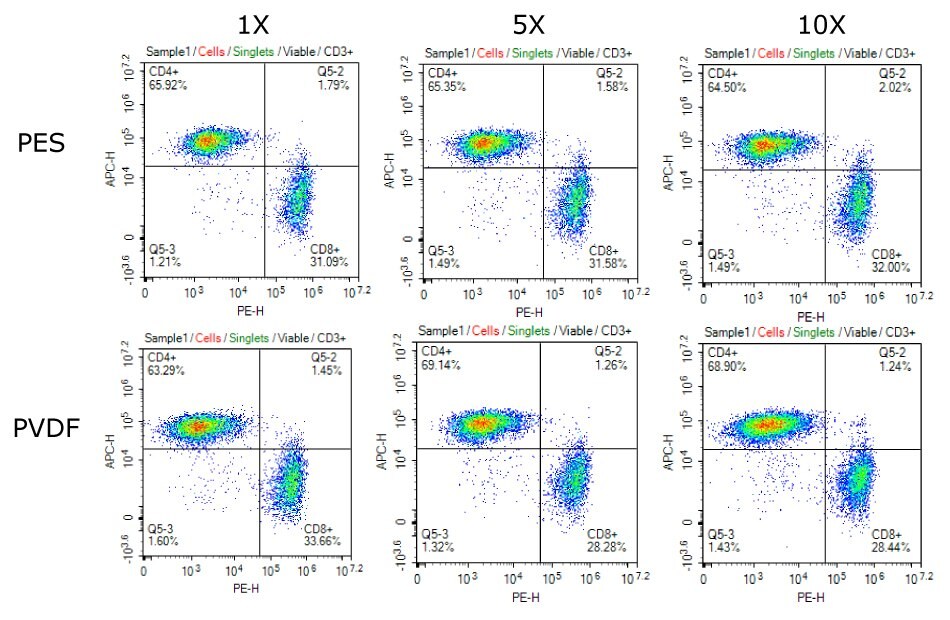
Figure 1.Consistent CD4+/CD8+ phenotype of T cells cultured in media filtered 1X, 5X, or 10X using Stericup® Quick Release PES and PVDF filtration systems.
Clarification of cell supernatant with lentivirus
Steriflip® and Stericup® filters were used to clarify crude lentivirus in cell supernatants before downstream concentration over multiple experiments. Input and output titers [8.9 to ≥2.8 x 106 infectious viral particles (ivp)/mL] were similar without loss after filtering (Figure 2).
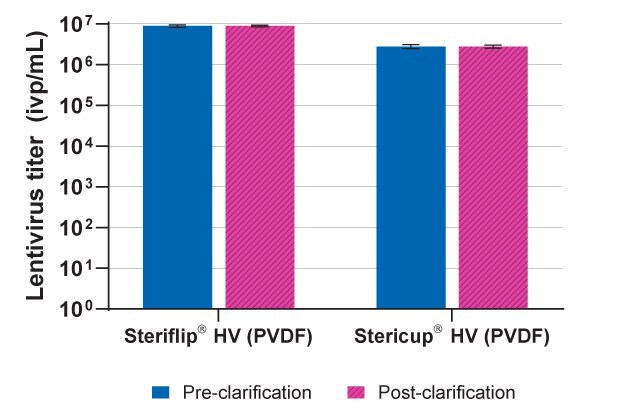
Figure 2.Viral lysate titers before and after clarification using 0.45 µm Steriflip® and Stericup® devices over multiple experiments.
Mycoplasma reduction
Mycoplasma (e.g., Acholeplasma laidlawii) has become widely recognized as the smallest bacterium thriving in cell culture. While there is currently no industry standard for testing or rating filters for mycoplasma clearance, the suitability of a consistently small, monodispersed, low aggregation bacterial challenge suspension using cultivation medium, incubation conditions, and challenge suspension preparation for achieving rigorous test conditions is crucial. The A. laidlawii challenge test follows the ASTM® F838-05 Standard Test for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration.3 Using these guidelines, an internally validated mycoplasma challenge test method for assessing the mycoplasma clearance performance of 0.1 µm and 0.22 µm sterilizing-grade filters was used to determine Log Reduction Values (LRV) for Stericup® filters containing 0.1 µm Millipore Express® PLUS PES as well as 0.22 µm Millipore Express® PLUS PES and Durapore® PVDF membranes (Figure 3). These sterilizing, hydrophilic membranes demonstrate high mycoplasma retention efficiency, ensuring mycoplasma are effectively reduced during filtration processes. Highest LRVs were obtained when using 0.1 µm rated Stericup® systems. These data demonstrate the utility of 0.1 µm Stericup® filters for mycoplasma-reduced sample processing.
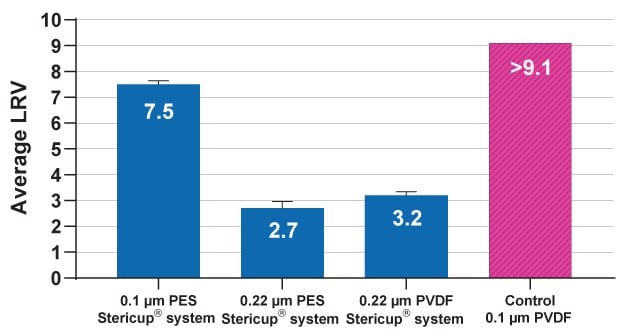
Figure 3.Log reduction values (LRV) of mycoplasma after 0.1 and 0.22 µm filtration.
Large volume filtration of media
Filtration is a critical step in preparing bacterial Luria Bertani (LB) bacterial broth for cultivation of Escherichia coli cultures and basal cell culture Dulbecco's Modified Eagle Medium (DMEM) supplemented with serum [DMEM with 10% fetal bovine serum (FBS)], particularly when scaling up to 10 liters. This process ensures the removal of contaminants including bacteria, fungi, and particulates which can adversely affect cell growth and experimental outcomes. We demonstrated the use of large volume capacity Stericap®-PLUS GP filter units in filtering Luria broth and DMEM with and without FBS (Figure 4). Such filtration systems help researchers achieve sterility when processing large volumes of culture media, thereby maintaining the integrity of the cell culture environment. This is essential for reproducibility in experiments, especially in cell culture and microbiological applications where even minor contamination can lead to significant variations in results.
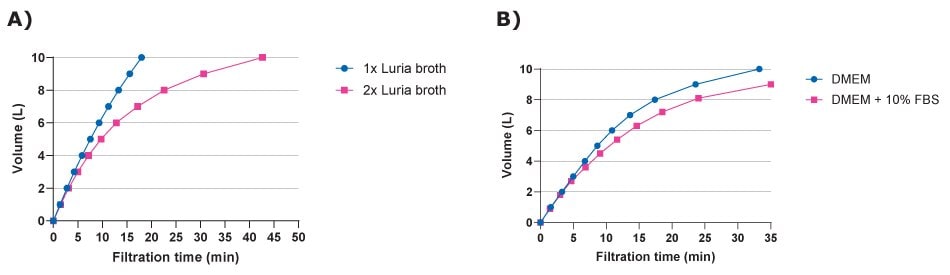
Figure 4.Large volume filtration of A) bacterial and B) cell culture media using Stericap®-PLUS GP vacuum filtration devices containing 0.22 µm PES Express® PLUS filters. High concentration media formulations (2X Luria broth) and serum-supplemented medium (DMEM + 10% FBS) required longer times to process compared to base media.
Steritop® Vacuum Filters (volumes <1L)
Receiver Bottles
Stericup® E and Steritop® E Eco-Friendly Vacuum Filters (volumes <1L)
Steriflip® Vacuum Filter Units (volumes <50 mL)
Sterile Millex® Syringe Filters (volumes 0.5 mL -100 mL)
Sterivex™ Pressure-Driven Filters (volumes <2 L)
Stericap® PLUS Vacuum-Driven Filters (volumes 5 – 10 L)
Steripak™ Pressure-Driven Filters (volumes <20 L)
References
如要继续阅读,请登录或创建帐户。
暂无帐户?