Removal of Percoll after Centrifugation
Because Percoll is non-toxic to biological materials and does not adhere to membranes, it is usually unnecessary to remove Percoll from the purified preparation. Cells can be transferred directly to cell culture systems, virus infectivity is unimpaired, and organelles can be used for metabolic studies without any effect caused by the gradient material.
The following methods can be used to eliminate the gradient material if desirable.
Washing (low-speed centrifugation)
Living cells can be separated from Percoll medium by washing with physiological saline (five parts saline to one part of Percoll cell suspension). The washing may be repeated two or three times and the cell collected between each washing step by centrifugation at 200 × g for 2-10 min. Studies with radioactively labeled Percoll (Table 2) have shown that no detectable residual Percoll is left adhering to cells washed in this way. Electron micrographs by Enerbäck et al. (Figure 17) and Schumacher et al. show cell preparations with no visible contaminating particles from the gradient material.
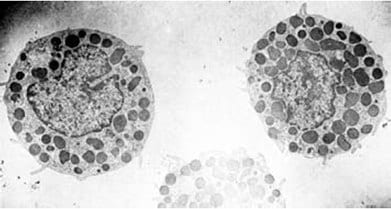
Figure 17. Electron micrograph of mast cells isolated by gradient centrifugation on Percoll (reproduced by kind permission of the authors and publisher).
Washing (high-speed centrifugation)
For viruses and subcellular particles which are too small to be pelleted by low speed centrifugation as described above, the biological material can be separated from coated silica particles by high speed centrifugation in a swinging bucket rotor or angle-head rotor. The undiluted fraction obtained from the first centrifugation run is placed in a centrifuge tube and spun in a swinging bucket rotor at 100,000 × g for 2 h, or 90 min in an angle-head rotor (100,000 × g) to pellet the Percoll. The biological material remains above the hard pellet of Percoll.
(Original work by Pertoft et al. reproduced by kind permission)
Other methods
Chromatography by gel filtration on Sephacryl™ S-1000 Superfine will separate Percoll from larger particles (e.g., subcellular particles), which are eluted in the void volume. Removal of Percoll from microsomal vesicles by gel filtration on Sephacryl S-1000 Superfine has been reported. The authors followed the elution pattern by assaying for the microsomal marker enzyme NADPH-cytochrome c reductase (Figure 18). The resulting microsomal fraction was examined by electron microscopy and found to be almost free from Percoll (less than 0.5% compared with the initial sample).
Preliminary experiments using electrophoresis to separate lysosomes and viruses from Percoll have been reported, but the methodology is difficult and results are often unpredictable (Pertoft, personal communication).

Figure 18. Gel filtration of microsomes obtained from gradients of Percoll containing 125I-labeled Percoll on Sephacryl S-1000 Superfine. Vo = void volume, Vt = total volume (reproduced by kind permission of the authors and publisher).
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?