A New Class of NHC-Based Pd Complexes for Challenging Cross-Couplings of Aryl Chlorides
A class of N-heterocyclic carbene (NHC) -based palladates, (NHC-H)2Pd2Cl6 (NHC = IPr, SIPr, IPent, IPr*, IPr*OMe and IPr#), was synthesized in excellent yields and characterized. Comparison of their catalytic activities in Suzuki-Miyaura coupling with other NHC-Pd-based precatalysts revealed comparable or superior activities, besides avoiding the use of “throw-away” stabilizing ligands. These precatalysts were demonstrated for Suzuki-Miyaura and Buchwald-Hartwig couplings with good substrate scope and isolated yields. In addition, trans-amidation coupling was also demonstrated.
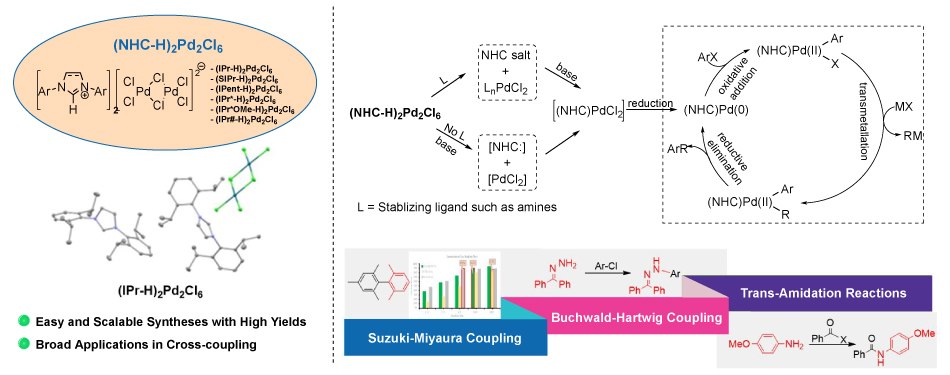
Figure 1. Structure, synthesis, and catalytic applications of (NHC-H)₂Pd₂Cl₆ precatalysts. This schematic shows a plausible activation pathway of (NHC-H)₂Pd₂Cl₆ precatalysts prior to the cross-coupling catalytic cycle. Applications include Suzuki–Miyaura, Buchwald–Hartwig, and trans-amidation reactions. Highlights note scalable synthesis and broad utility.
Past Solutions, Present Challenges in Pd–NHC Catalysis
N-heterocyclic carbenes (NHCs) have emerged as a distinctive and powerful class of ligands in cross-coupling chemistry, particularly for the development of palladium-based precatalysts.1–15 The majority of the studies in this area were focused on palladium-catalyzed processes with significant contributions from the groups of Nolan,10,16–18 Organ,19,20 Glorius,15 Szostak,21–24 Hazari,16,25–28 and others29–33 (Figure 2). To stabilize the highly reactive 12-electron NHC–Pd(0) species formed during catalysis, chemists have adopted the use of “throw-away” stabilizing ligands such as allyl, crotyl, cinnamyl, 3-chloropyridyl, and t-butyl indenyl. However, incorporating these ligands onto palladium remains a nontrivial challenge.1
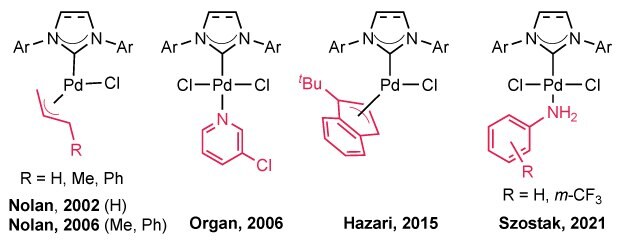
Figure 2.Examples of reported NHC-Pd precatalysts with “throw-away” stabilizing ligands (red) as reported by Nolan (2002, 2006), Organ (2006), Hazari (2015), and Szostak (2021).
Among modern NHC-based catalytic systems involving monoligated Pd-NHC complexes with spectator/throw-away ligands, a notable breakthrough came in 2002 when Nolan introduced a series of (NHC)Pd(allyl)Cl precatalysts stabilized by allyl groups.34 Building on this, the group later developed (NHC)Pd(R-allyl)Cl complexes in 2006, featuring Me or Ph substitutions on the allyl ligand, which allowed room-temperature activation compared to the 70°C required for the original system.35 While these precatalysts were effective in cross-coupling reactions, they were prone to forming inactive bridging Pd(I)–allyl dimers during activation, negatively impacting catalytic efficiency. However, bulkier substituents on the allyl group or ligand backbone helped mitigate dimer formation and improve activation.26,36–38
Toward Scalable and Ligand-Free NHC–Pd Precatalysts
While Pd–PEPPSI precatalysts are accessible in good yields, their synthesis typically requires relatively harsh conditions (80°C in 3-chloropyridine for 16 hours) and time-intensive workups, limiting their scalability for bulk applications.
In 2015, the Hazari group introduced a new class of NHC–Pd precatalysts stabilized by η³-1-tBu-indenyl ligands.25,28 Unlike their R-allyl counterparts, these complexes were designed to suppress the formation of inactive Pd(I) dimers formed via comproportionation and demonstrated strong performance in Suzuki–Miyaura and Buchwald–Hartwig couplings. However, large-scale synthesis remains challenging, often involving multi-step procedures and the difficult incorporation of the t-butyl group into the indenyl framework. The latter step raises particular safety concerns due to the use of tBuLi.25,40 More recently, Szostak and coworkers reported a series of aniline-stabilized NHC–Pd precatalysts, (NHC)Pd(AN)Cl₂ (AN = aniline or m-CF₃ aniline), showcasing their broad utility in cross-coupling reactions.21,23
Although many of these NHC-Pd-based precatalysts containing stabilizing ligands are widely utilized in both academic research and industrial applications, their limited Pd atom economy and (relatively) complex manufacturing processes restrict broader adoption due to high cost and scalability challenges. Developing a one-pot, high-yield synthesis of NHC–Pd precatalysts could significantly advance the field.
Recently, stabilizing-ligand-free NHC–Pd precatalysts have garnered attention, with contributions from Nolan, Szostak, and Gooßen (Figure 3A).24,41–45 Complexes such as [Pd(NHC)(μ-Cl)Cl]₂ have shown promise in Suzuki–Miyaura and Buchwald–Hartwig couplings but often rely on less practical reagents like Ag₂O or corrosive HCl, limiting broader applicability. 24,41,43,44
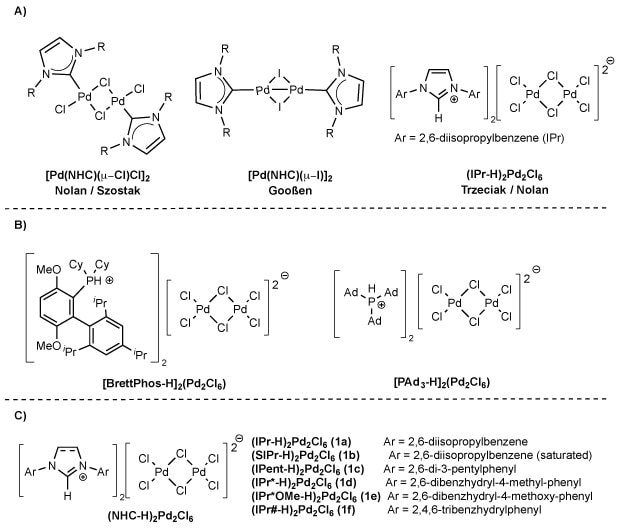
Figure 3.Examples of stabilizing-ligand-free NHC-Pd precatalysts (A), monodentate phosphine palladates (B), and NHC-Pd precatalysts studied (C). Includes control studies on (NHC-H)₂Pd₂Cl₆ activation with key reactions (70–100% yields) and a proposed catalytic cycle showing oxidative addition, reduction, and transmetalation. Inset: 3D model of Pd-butylamine complex.
Herein, we present a new class of stabilizing-ligand-free NHC–Pd precatalysts, (NHC-H)2Pd2Cl6 (Figure 3C), synthesized under mild, scalable conditions and demonstrating excellent catalytic performance. While similar complexes were reported by Trzeciak in 2015 via a two-step synthesis (60% overall yield),46,47 and by Nolan in a mechanistic study,45 neither group explored their application in cross-coupling. Related phosphine-based systems, such as [P-H]₂Pd₂Cl₆ (Figure 3B), have also been used in cross-coupling,48 though they rely on bulky monodentate ligands like BrettPhos or CataCXium.
Results and Discussion: Efficient Synthesis and Characterization of (NHC-H)₂Pd₂Cl₆ Complexes
Unlike the synthesis of (IPr-H)2Pd2Cl6 reported by the Nolan group, which required elevated temperature (60oC) in a flammable organic solvent (acetone),45 the (NHC-H)2Pd2Cl6 complexes reported herein were all synthesized under much milder conditions (reacting Na2PdCl4 with NHC salts in methanol/water at room temperature). All products were isolated in excellent yields after a simple filtration (>80% isolated, Scheme 1). Four of these NHC palladates, namely, (IPr-H)2Pd2Cl6 (1a), (SIPr-H)2Pd2Cl6 (1b), (IPent-H)2Pd2Cl6 (1c), and (IPr#-H)2Pd2Cl6 (1f), were scaled up to >20 grams without any yield loss, demonstrating the scalability of the process for large-scale applications and consequently cost savings and sustainability.
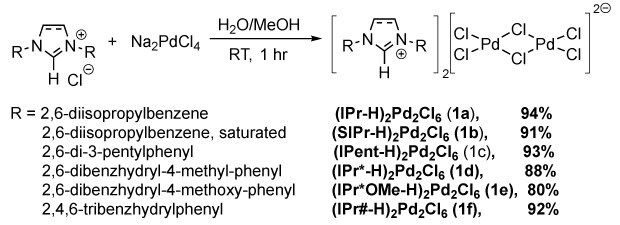
Scheme 1.Synthesis of (NHC-H)2Pd2Cl6 (1a – f). (NHC-H)₂Pd₂Cl₆ complexes were prepared by reacting NHC salts with Na₂PdCl₄ in water/methanol at room temperature for 1 hour. Variations in NHC ligands (1a–f) yielded 80–94%, producing Pd dimers with square planar geometry coordinated by two NHC ligands and four chlorides.
All the products were fully characterized with 1H NMR, 13C NMR and elemental analysis. (IPr-H)2Pd2Cl6 (1a), (SIPr-H)2Pd2Cl6 (1b) and (IPr#-H)2Pd2Cl6 (1f) were confirmed with single-crystal X-ray crystallography.
Activation Pathways and Catalytic Performance of NHC-Pd Complexes
In principle, these complexes need to be activated cooperatively on both [NHC-H]+ and [Pd2Cl6]2- motifs. We carried out two controlled reactions: i) reacting (IPr*-H)2Pd2Cl6 (1d) with base (NaOtBu) first, followed by the addition of amine (nBuNH2) (reaction 1 in Scheme 2A); and ii) reacting (IPr*-H)2Pd2Cl6 (1d) with amine first, followed by base (reaction 2 in Scheme 2A). Both reactions gave precatalyst 2 in good yields (reaction 1, 70%; reaction 2, 90%). With these observations, we were confident that, with the presence of an amine, our (NHC-H)2Pd2Cl6 precatalysts can be activated to form 2 as the active precatalyst in cross-coupling reactions. In addition, precatalyst 2 was utilized for a Buchwald-Hartwig reaction (reaction 3 in Scheme 2A) with quantitative conversion. Combining all these findings, we believe that these (NHC-H)2Pd2Cl6 precatalysts can be utilized as the effective catalysts in cross-coupling reactions with the proposed mechanism in Scheme 2B.
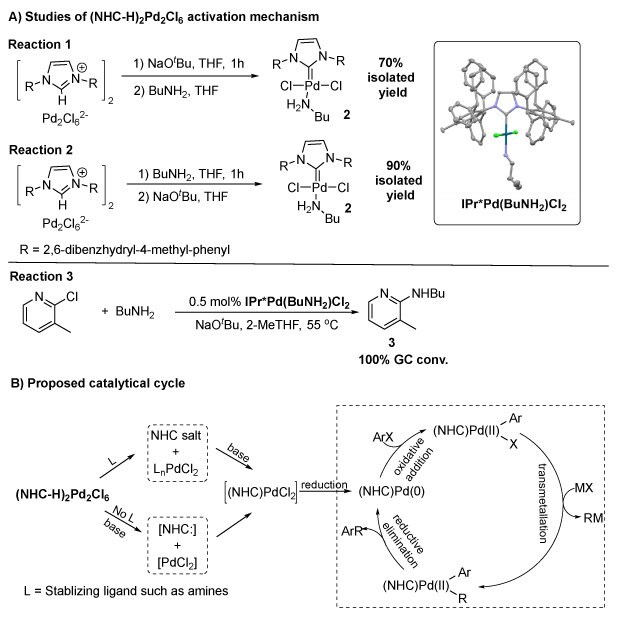
Scheme 2.Control studies on (NHC-H)2Pd2Cl6 activation mechanism (A) showing three key reactions with isolated yields of 70–100%, and (B) the proposed catalytic cycle illustrating oxidative addition, reduction, and transmetalation steps involving NHC salts and Pd intermediates. The inset features a 3D model of the activated Pd complex coordinated with butylamine and chloride.
Given the structural and mechanistic similarities between our (NHC-H)2Pd2Cl6 precatalysts and established systems such as the Pd–PEPPSI series, (NHC)Pd(R-allyl)Cl, and (NHC)Pd(tBu-Ind)Cl, we anticipated comparable, if not superior, performance in cross-coupling reactions. We were particularly interested in whether these commonly used “throw-away” stabilizing ligands (e.g., 3-chloropyridine, tBu-indenyl) could be entirely omitted without compromising catalytic activity.
To explore the necessity of “throw-away” stabilizing ligands, we compared our (IPent-H)2Pd2Cl6 (1c) with two commercial precatalysts, Pd–PEPPSI–IPent and (IPent)Pd(tBu-Ind)Cl, in a challenging Suzuki–Miyaura reaction between chloromesitylene and 2,6-dimethylphenylboronic acid (Figure 4). While all three achieved >90% conversion, reaction rates varied notably. Our catalyst (1c) completed the reaction in ~10 h, faster than Pd–PEPPSI–IPent (~20 h), suggesting the stabilizing 3-chloropyridine may impede turnover. The tBu-Ind-stabilized precatalyst showed the fastest rate (~6 h). While differences were moderate, these results support the idea that eliminating stabilizing ligands can maintain activity while improving atom economy and simplifying downstream purification.
Broad Applications in Cross-Coupling Reactions
We then expanded our application studies with respect to the substrate scopes and types of cross-coupling reactions (i.e., Suzuki-Miyaura coupling, Buchwald-Hartwig coupling, and trans-amidation coupling). While cross-coupling reactions of aryl bromide catalyzed by NHC-Pd-based precatalysts were well documented in literature, we were particularly interested in the cross-coupling chemistry of relatively challenging aryl chlorides.
In the Suzuki–Miyaura coupling reactions (Figure 4), using the previously demonstrated conditions (1–2 mol% Pd, KOH as base), sterically hindered aryl–aryl (sp²–sp²) couplings performed well, with isolated yields exceeding 80%. For example, using (IPent-H)2Pd2Cl6 (1c), product 4a was obtained in 84% yield. Similarly, (IPr*-H)₂Pd₂Cl₆ (1d) delivered 4a in 80% yield and 4b in 84%.
In contrast, for less hindered substrates such as product 4c, (IPr-H)2Pd2Cl6 (1a) gave excellent results (81% yield), although this same precatalyst gave negligible conversion (<10%) for the more hindered 4a and 4b. Notably, nitrogen-containing substrates like pyridine were also well tolerated, as demonstrated by the 91% isolated yield of 4d.
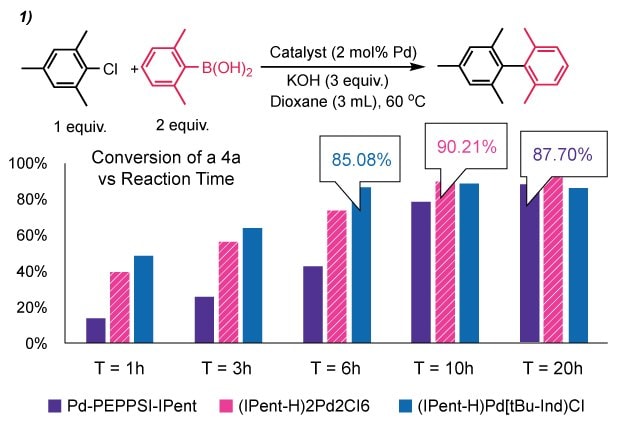
Figure 4.1.Suzuki-Miyaura coupling using (NHC-H)2Pd2Cl6 precatalysts, benchmarking (IPent-H)Pd2Cl6 against Pd-PEPPSI-IPent and (IPent)Pd(tBu-Ind)Cl.
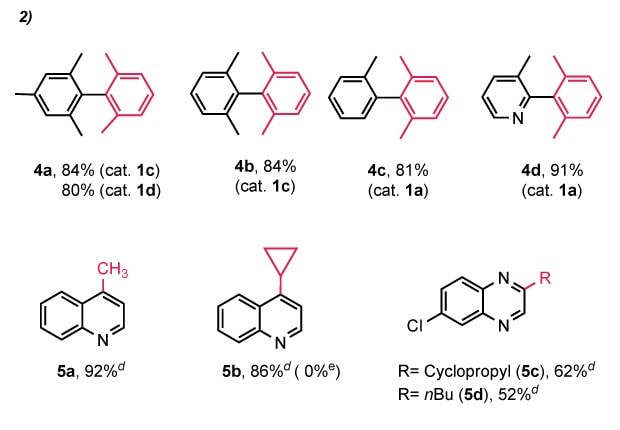
Figure 4.2.Substrate scope aReaction conditions: (Het)Ar-Cl (0.5 mmol, 1.0 equiv.), boronic acid/ester (1.5–2 equiv.), KOH (2.0 equiv.), [NHC-H]₂Pd₂Cl₆ (1.0 mol% Pd), solvent, 55 or 60 °C; bcatalyst loading: 2.0 mol% Pd, solvent; dioxane, 60 °C, GC conversion, cisolated yield; dfor sp²–sp³: (IPr#-H)₂Pd₂Cl₆ 1f (1.0 mol% Pd), solvent; dioxane, 90 °C; control; eno catalyst.
Although sp²–sp³ cross-coupling reactions using NHC–Pd catalysts have been demonstrated with Pd–PEPPSI precatalysts in Negishi and Feringa–Murahashi systems, Suzuki–Miyaura-type sp²–sp³ couplings remain less explored with NHC–Pd systems.⁴⁹,⁵¹-⁶⁰ Nevertheless, we successfully applied our (NHC-H)2Pd2Cl6 catalysts in these challenging reactions, achieving strong performance within a limited substrate scope.
Using (IPr#-H)₂Pd₂Cl₆ (1–2 mol% Pd loading), coupling of 4-chloroquinoline with methylboronic acid or cyclopropylboronic acid yielded products 5a and 5b in 92% and 86% isolated yields, respectively. No product was formed in the absence of a catalyst.
Additionally, stepwise coupling was observed for Ar-Cl with two chloride groups of differing reactivity, such as 2,6-dichloroquinoxaline. Mono-coupled products 5c and 5d were obtained in good yields (62% and 52%, respectively), with the second chloride remaining untouched.
NHC-Pd-catalyzed Buchwald-Hartwig couplings of aryl chlorides have been broadly demonstrated in literature with significant contributions from the groups of Nolan,17,34,35 Organ,20,50 Hazari,18,25,28 Szostak,21,22,44,58 and many others.5,61 Without too much surprise, (NHC-H)2Pd2Cl6 also worked successfully with broad substrate scopes and excellent isolated yields (Figure 5). Select coupling of primary amines (6a-f) and secondary amines (6g and 6h) was generally observed. Both aryl amines and alkyl amines were successfully utilized in the coupling reactions catalyzed by (NHC-H)2Pd2Cl6 precatalysts. Worthwhile to note, primary hydrazones, i.e., benzophenone hydrazone, reacted with aryl chlorides smoothly, even in the case of the sterically hindered 2,6-dimethylchlorobenzene, with excellent isolated yields (6i, 93%; 6j, 94%, respectively).
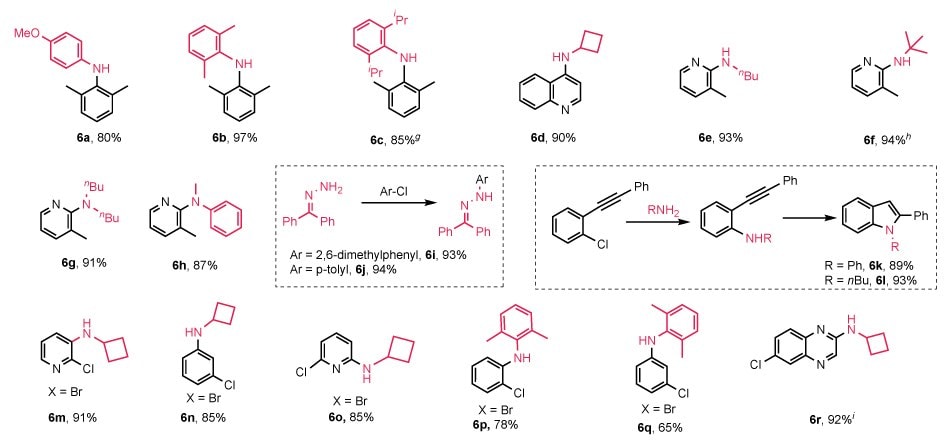
Figure 5.Buchwald–Hartwig coupling study using (NHC-H)2Pd2Cl6 precatalysts, showing broad substrate scope, high yields, and selective halogen reactivity. fReaction conditions: Ar–X (1.0 mmol), amine (1.5 equiv.), NaOtBu (1.5 mmol, 1.5 equiv.), [IPr-H]₂Pd₂Cl₆ (1d 0.5 mol%), 2-MeTHF (1 mL), 55 °C; run at g105 °C; hisolated as HCl salt; iK₃PO₄ (3 equiv.), dioxane, 100 °C.
Our (NHC-H)2Pd2Cl6 precatalysts also enabled efficient cascade reactions and exhibited notable haloselectivity. Indole derivatives 6k and 6l were obtained in excellent yields via cascade Buchwald–Hartwig coupling of primary amines followed by hydroamination of a C–C triple bond.⁶²
Notably, the catalysts demonstrated excellent selectivity between halogens on polyhalogenated substrates. Selective amination occurred preferentially at the Br position over Cl in aryl halides (Ar–X), affording products 6m–6q in good to excellent yields. This selectivity is largely due to the reactivity difference between the Br and Cl groups on the substrates. Similarly, product 6r was obtained successfully from a selective coupling reaction on the relatively more active Cl group of 2,6-dichloroquinoxaline and cyclobutylamine (6r, 92%).
Lastly, we briefly expanded the applications of our (NHC-H)2Pd2Cl6 into Pd-catalyzed trans-amidation couplings.58 Both ester trans-amidation and amide trans-amidation worked smoothly with moderate to excellent isolated yields. For instance, in Figure 6, product 7a was isolated in good to excellent yields from both trans-amidation reactions (ester trans-amidation, 84%; amide trans-amidation, 91%). However, when the alkyl ester or amide was used as the electrophile, the reaction turned out to be much more sluggish with moderate isolated yields (7b, 62%; 7c, 40%). Boc-protected amino acid ester was well tolerated and utilized for ester trans-amidation with good yield (7c, 40%).
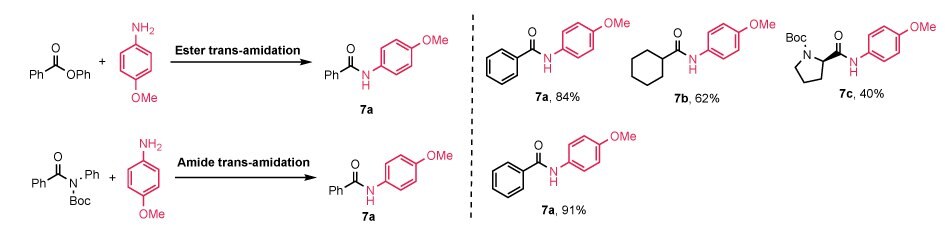
Figure 6.Use of (NHC-H)2Pd2Cl6 precatalysts in ester and amide trans-amidation couplinj, delivering moderate to excellent yields and tolerating Boc-protected substrates. jReaction conditions: Aniline (0.5 mmol, 2.0 equiv.), tertiary amide or Aryl benzoate (0.25 mmol, 1.0 equiv.), K2CO3 (0.75 mmol, 1.5 equiv.), (IPr*-H)₂Pd₂Cl₆, (1d, 3 mol%), DME (1 mL), 100 °C.
In conclusion, we have developed and characterized a class of N-heterocyclic carbene (NHC)-based palladates, (NHC-H)2Pd2Cl6 (NHC = IPr, SIPr, IPent, IPr*, IPr*OMe and IPr#) that operate without the need for stabilizing “throw-away” ligands. Mechanistic studies and direct benchmarking against leading commercial NHC–Pd precatalysts revealed comparable or superior catalytic activity. These ligand-free systems performed exceptionally well across diverse and industrially relevant cross-coupling chemistries, including Suzuki–Miyaura, Buchwald–Hartwig, and trans-amidation couplings, offering broad substrate scope, excellent isolated yields, and promising potential for sustainable large-scale applications.
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?