Bis(2-methoxyethyl)aminosulfur Trifluoride (Deoxo-Fluor®)
Originally reported by Lal and coworkers in 1999,1 bis(2-methoxyethyl)aminosulfur trifluoride (Deoxo-Fluor®) has shown remarkable utility in organic synthesis as a thermally stable alternative to (diethylamino)sulfur trifluoride (DAST). Deoxo-Fluor® can easily convert alcohols to alkyl fluorides, aldehydes and ketones to gem-difluorides, and carboxylic acids to acid fluorides or trifluoromethyl derivatives (Scheme 1).2
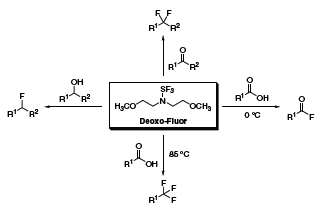
Scheme 1.
A recent report showed the use of Deoxo-Fluor® in the synthesis of a 2-azabicyclo[2.1.1]hexane analogue of 4-fluoroproline
(Scheme 2).3 Although the reaction generally proceeds with inversion at the chiral carbon, the configuration is retained in this constrained substrate due to neighboring-group participation of the amide group.
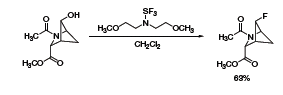
Scheme 2.
Deoxo-Fluor® can be used to effect a wide array of additional transformations. One recent example highlights its use in the syntheses of a series of chiral C2 bis-oxazoline ligands (Scheme 3).4 The chemoselectivity of Deoxo-Fluor® was superior to other reagents, including DAST, and was tolerant of steric and electronic variations within the ligands. Furthermore, the Deoxo-Fluor® protocol also allowed the majority of the ligands to be purified without requiring chromatography.
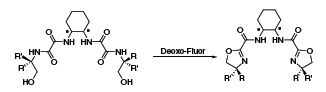
Scheme 3.
Gunda Georg at the University of Kansas has reported the use of Deoxo-Fluor® in a one-flask protocol to convert acids to amides and peptides or Weinreb amides (Scheme 4).5 The reaction proceeded under mild conditions and provided the desired products in high yields with facile purification.
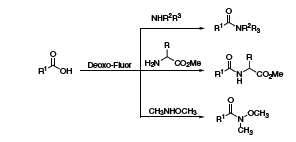
Scheme 4.
The Kangani group has devoted several publications to one-pot transformations achievable with Deoxo-Fluor®.6 Generally, the reactions begin with the conversion of a carboxylic acid to an acid fluoride, which is then reacted with various nucleophiles to produce oxazolines, aldehydes, ketones, benzoxazoles, oxadiazoles, acyl azides or nitriles (Scheme 5).
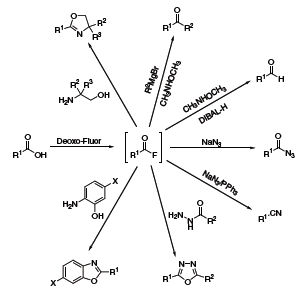
Scheme 5.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?