Optimal Water Purification for Silica-Sensitive Applications
Section Overview
- Problems Caused by Silica in Water
- Water Purification for Silica-sensitive Applications
- Efficacy of RO on Silica Removal from Water
- Efficacy of EDI on Silica Removal from Water
- Efficacy of IEX Resins on Silica Removal from Water
- Studies Assessing the Efficacy of Water Purification Technologies in Removing Silica
- Selecting a Water Source for Silica-sensitive Applications
- Importance of Water Quality for Silica-sensitive Applications
- Related Products
Problems Caused by Silica in Water in the Microelectronics Industry and in Laboratory Instruments
The presence of silica in water can cause problems for many industries, research areas and testing labs. Silica contamination of water can lead to silica coating, costly cleaning, and inaccurate results. Consequently, the removal of silica from water is a critical issue.
In the microelectronics industry especially, many applications are known to be silica sensitive. For example, the formation of silica deposits on wafers can lead to expensive chip failures. In these and other labs, instruments such as testing chambers (e.g., ISO 4892), healthcare sterilizers and autoclaves (e.g., ISO 17665-2006, ISO 17665-2:2009) may require water of good quality with a low level of silica to ensure optimal performance.
Water Purification for Silica-Sensitive Applications
In this article, we analyze the efficiency of various water purification technologies, alone and in combination, in removing silica from water. Performances of reverse osmosis (RO), electrodeionization (EDI) and ion-exchange (IEX) resins are assessed. The suitability of Milli-Q® water purification systems to deliver high-quality, low-silica water over time is also demonstrated. The water produced by these systems supports quality expectations of the microelectronics and healthcare industries and is compliant with norms and standards for pure and ultrapure water regarding silica content to meet customers’ application requirements.
The Role of Water Purification Technologies and System Design in Avoiding and Removing Silica Contamination in Water
Laboratory water purification systems, used to produce water of the required quality, need to be well-designed and validated to efficiently remove the large quantities of silica from feed water and to prevent silica release (leaching) from the system itself. Indeed, the efficiency of the individual water treatment methods, and of the purification system as a whole, can be negatively impacted by the materials used in the system and by the incorrect combination of the purification technologies (RO, EDI and IEX resins) as each technology has a specific effect on silica removal.
Silica Structures and their Measurement in Water
The chemistry and behavior of silica is complex and depends on its environment. In solution, silica exists under various forms:
- Monosilicic acid, Si(OH)4 (very weak acid, pKa 9.84)
- Polysilicic acids
- Colloidal silica particles
Studies investigating the dissolution of silica species have revealed that their chemical structures depend on factors such as temperature, degree of crystallinity, and pH (Figure 1).1
![<b>A.</b> Structures of hydrated silicic acid H<sub>4</sub>SiO<sub>4</sub> (H4) and its hydrated dominant-associated base H<sub>3</sub>SiO<sub>4</sub><sup>−</sup> (H3) in aqueous solution. Distribution of H4 and H3 species in aqueous solution as a function of pH according to the dissociation constants of pKa<sub>1</sub> = 9.84 and pKa<sub>2</sub> = 13.2 at 298 K and 0 M ionic strength condition. Silicon, oxygen, and hydrogen atoms are represented in yellow, red, and white, respectively. Adapted from Wang W et al. 2019.<sup>2</sup> <b>B.</b> Equations of acid-basic equilibrium of silica species in aqueous solution.<sup>3</sup> General formula of silicates: [SiO<sup>(4−2x)−</sup><sub>4−x</sub>]<sub>n</sub> where 0 ≤ x < 2. Acid-basic equilibria of silica species in aqueous solution](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/materials-science-and-engineering/microelectronics-and-nanoelectronics/structures-of-hydrated-silicic-new/structures-of-hydrated-silicic-new.jpg)
Figure 1.A. Structures of hydrated silicic acid H4SiO4 (H4) and its hydrated dominant-associated base H3SiO4− (H3) in aqueous solution. Distribution of H4 and H3 species in aqueous solution as a function of pH according to the dissociation constants of pKa1 = 9.84 and pKa2 = 13.2 at 298 K and 0 M ionic strength condition. Silicon, oxygen, and hydrogen atoms are represented in yellow, red, and white, respectively. Adapted from Wang W et al. 2019.2 B. Equations of acid-basic equilibrium of silica species in aqueous solution.3 General formula of silicates: [SiO(4−2x)−4−x]n where 0 ≤ x < 2.
The complexity of the various chemical structures of silica makes it difficult to be removed from water. Table 1 describes the potential purification technologies for silica decontamination. Table 2 outlines the analytical methods that can be used to measure silica content in water according to its form in aqueous solution.
Efficacy of Reverse Osmosis (RO) on Silica Removal from Water
RO membranes reject 97‒99% of soluble and insoluble silica, however, bulk quantities of silica in feed water can result in membrane scaling, fouling and reduced performance. To avoid scaling over a wide pH range and to prolong membrane lifetime, the quality of the feed water should be considered in a comprehensive manner and adequate pretreatment steps should be implemented, as is generally done in industrial plants. Furthermore, RO treatment alone is frequently insufficient and cannot guarantee quality consistency and low silica levels for silica-sensitive applications and instruments, such as for autoclaves and downstream ultrapure water polishing systems.
Efficacy of Electrodeionization (EDI) on Silica Removal from Water
EDI is an electrochemical method combining IEX resins, selective anionic and cationic semi-permeable membranes, and the application of a small electrical current or voltage. Thanks to the applied electrical field, anions and cations migrate to oppositely charged electrodes, resulting in their concentration and removal via waste channels. The resin beads facilitate the mass transfer of ions, including weakly-ionized species, such as silicic acid and silicate ions (Figure 1). This shifts the equilibrium towards silicate ions, consequently allowing the removal of SiO2 as well (Figure 1b). Due to water splitting, the IEX resin bed is continuously regenerated, which avoids the needs for chemical regeneration procedures and associated waste.
Efficacy of Ion-Exchange (IEX) Resins on Silica Removal from Water
Strong base IEX resins (hydroxyl ions) have been shown to remove silicates, however resins have limited capacity. When exhausted, all resin binding sites are occupied and must be regenerated using caustic soda. This procedure leads to additional costs, time loss, and waste disposal (environmental impact). IEX resins are therefore not ideal technologies for the first step of water purification as many ions need to be removed from feed water. Moreover, depending on the relative affinity values of resins for different ions, they do not allow the efficient continuous removal of most ions simultaneously. IEX resins are also sensitive to fouling from colloidal silica deposit.
Water purification systems that exclusively use IEX resins, such as service deionization (SDI) bottles, can lead to silica leakage due to the difference in ionic affinity of silica to resins. This can lower the efficiency of downstream water polishing systems and instruments. To avoid this problem, modern Milli-Q® water purification systems employ a combination of technologies that are designed to prevent this breakthrough phenomenon and guarantee optimal protection and performance of downstream instruments. Within Milli-Q® systems, IEX resins are employed preferably at the end of the purification process, in the polishing steps, to produce ultrapure water.
Detection of Silica in Water and Risk of Silica Breakthrough with Ion-Exchange Resins
Silica is weakly ionized in water, consequently it cannot be easily detected using conductivity. Studies show silica to be an early-breakthrough element from IEX resins,4 similar to boron. Analysis identifies that silica is released before the drop in resistivity is detected (Figure 2). This means that water quality can be affected without the user being aware, which can be detrimental to experiments and instruments.
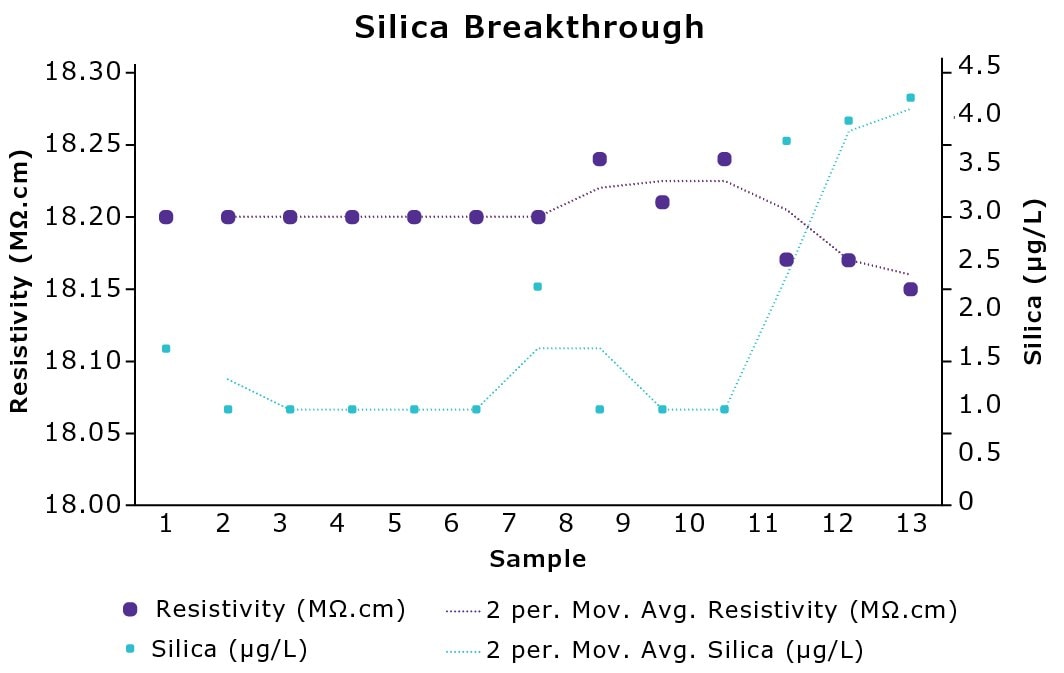
Figure 2.Evolution of silica and resistivity over time showing silica breakthrough from IEX resins in experiments performed internally.
Studies Assessing the Efficacy of Water Purification Technologies in Removing Silica
Study 1: Silica Removal According to Water Purification Technology
Previous studies performed in Merck Lab Water Solutions laboratories showed that silica is most efficiently removed by a combination of water purification technologies, specifically, an RO membrane followed by an EDI module (Table 3).3 RO in conjunction with EDI technology delivered water of high resistivity, a low, stable level of silica, and showed a significantly reduced risk of breakthrough. As expected, IEX resins produced high resistivity water only for a very limited period before exhaustion, and important breakthrough of silica occurred before resistivity was observed to drop (as in Figure 2). Furthermore, resin regeneration leads to defects (i.e., molecular structure modifications of beads, resin degradation, etc.) that cause variations in water quality.
Study 2: Silica Levels after Consecutive Water Purification Steps in a Modern Water Purification System
A series of internal analyses was performed to evaluate the level of silica in pure and ultrapure water obtained from a Milli-Q® water purification system that includes RO, EDI and IEX technologies. The level of total silica was measured in tap water, RO water, EDI water, and IEX-polished water drawn at the point-of-dispense (referred to as ultrapure water) on two different days using a GF-AAS PerkinElmer® AAnalyst™ 600 instrument (PerkinElmer® Life and Analytical Sciences, Shelton, CT).
Results
Tap water contained around 16.8 mg/L of silica on day 1. The improvement in water quality after the RO membrane is shown in Figure 3. RO removed 99% of silica leading to a total silica value of 169 µg/L (day 1). The EDI module allowed to displace the silicic acid equilibrium reaching a total silica value < 2 µg/L. Finally, the polishing step, which included IEX resins to remove trace levels of charged molecules, led to a decrease of silica content that reached below the method limit of detection (1 µg/L). A second set of measurements (day 2) led to similar outcomes.
These results indicate that the RO membrane and the EDI module are crucial to remove a prominent part of silica content from tap water. Moreover, the ability to reach a level of silica below 10 µg/L prolongs the lifespan of downstream IEX resin-containing polishing packs that are responsible for removing trace levels of contaminants.
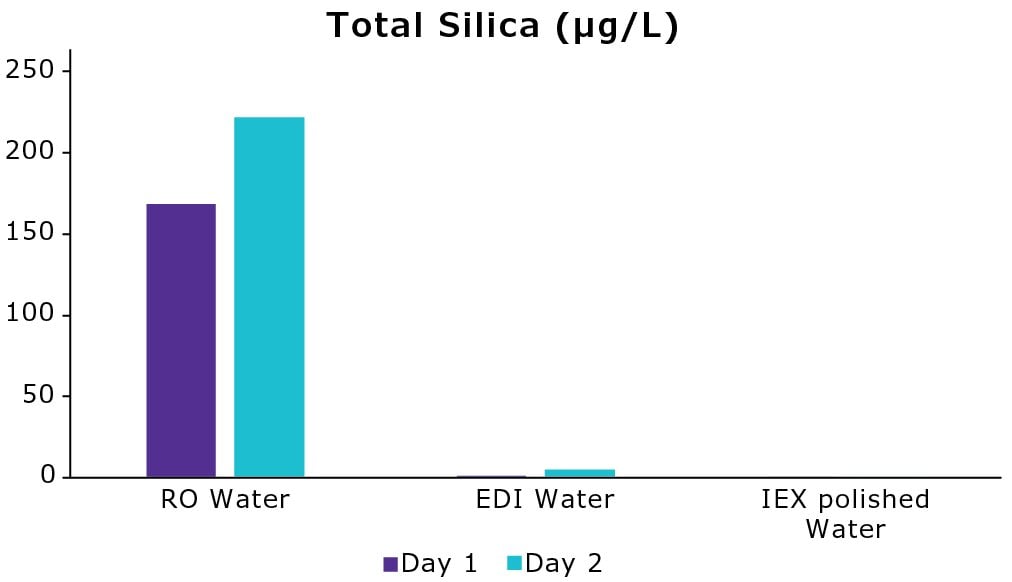
Figure 3.Evolution of total silica content (µg/L) in water on two different days after treatment by the RO membrane, the EDI module, and a polishing cartridge containing IEX resins within a water purification system (Milli-Q® ultrapure water system).
Study 3: Benefits of Modern Water Purification Systems
To confirm the suitability of a modern water purification system for laboratories where ultrapure water with low silica levels is required by standards/norms, samples of water delivered by a Milli-Q® IQ 7003 water purification system were analyzed (Figure 4). Total silica concentration was measured using GF-AAS.
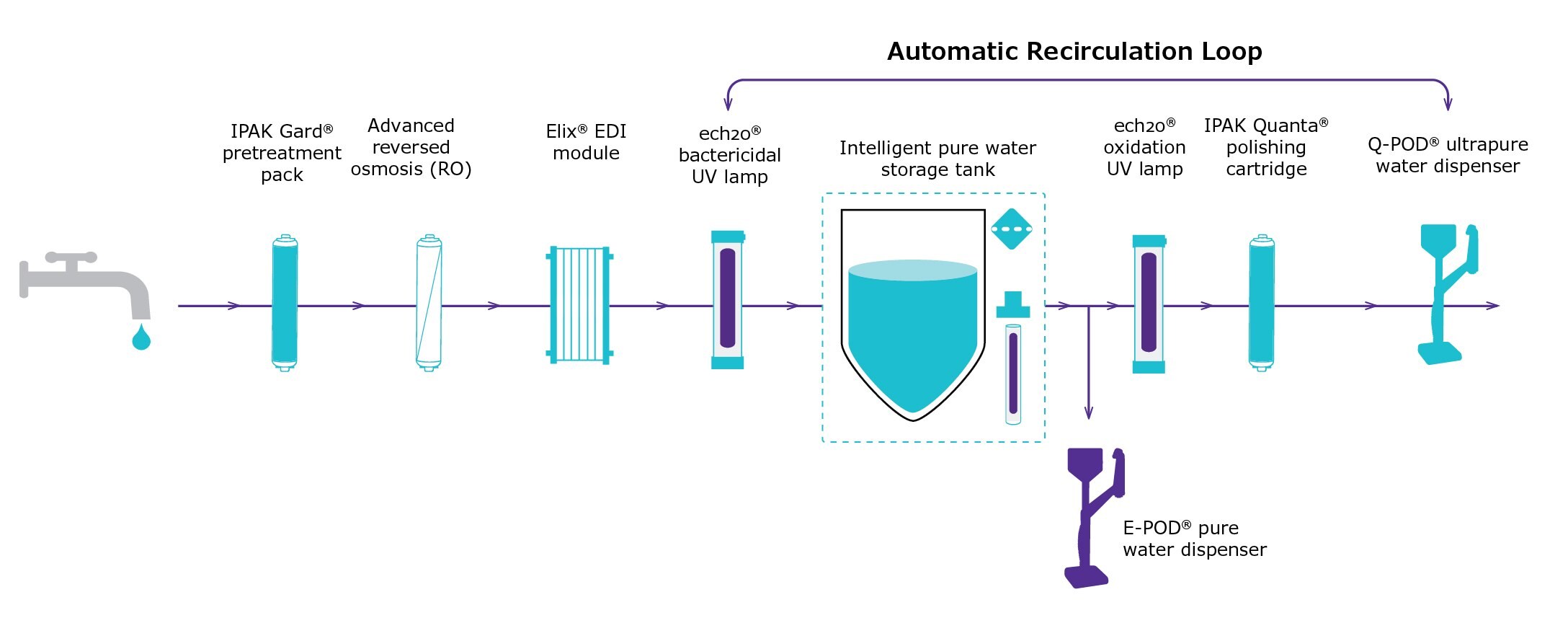
Figure 4.Scheme illustrating the combination of technologies in a Milli-Q® IQ 7003 pure and ultrapure water system. In this study, ultrapure water was withdrawn at the Q-POD® ultrapure water dispenser equipped with a Millipak® 0.22 μm final filter. An E-POD® pure water dispenser can also be installed immediately after the storage tank.
Results
The performance of the modern water purification system was evaluated in our laboratories. Analytical results of the ultrapure water delivered by the Milli-Q® IQ system revealed that silica content was below the method level of detection of 1 µg/L (Figure 5). As demonstrated in previous studies,3 the combination of RO and EDI efficiently removes silica and reaches lower levels of silica contamination in a stable manner compared to with RO or IEX alone. Water delivered by the Milli-Q® IQ system meets or exceeds the norms and standards of interest for ultrapure water (Table 4).
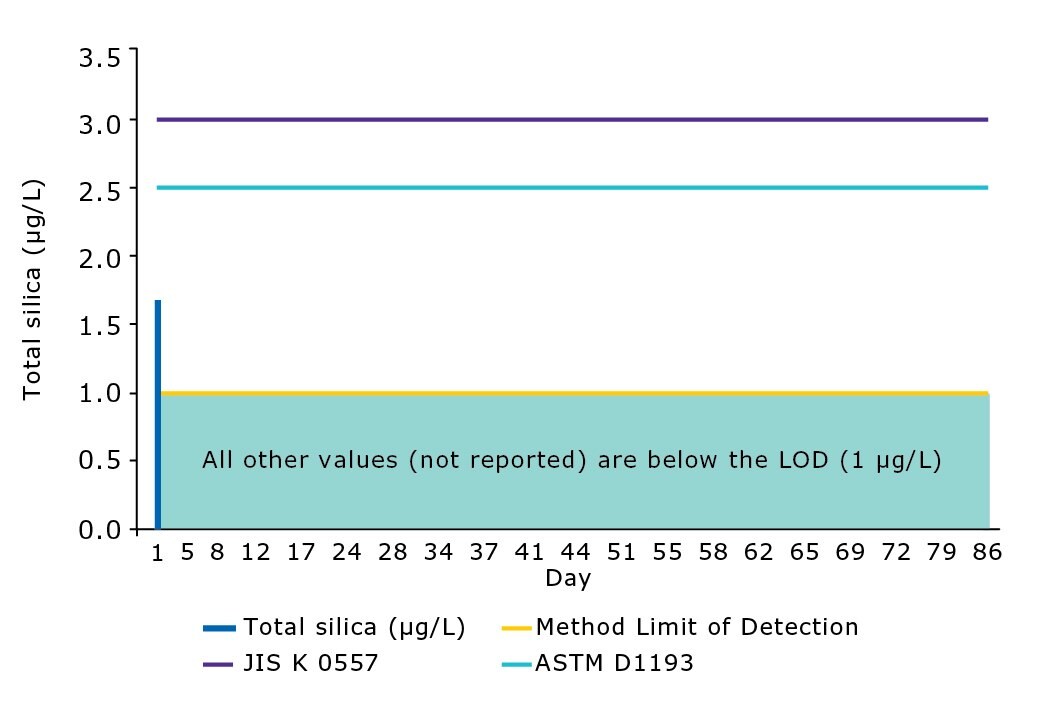
Figure 5.Silica levels in ultrapure water delivered by a Milli-Q® IQ 7003 system (Q-POD® dispenser with Millipak® final filter). All values after day 5 are below the method limit of detection (LOD). ASTM D1193-06 (2011) – Standard Specification for Reagent Water Type 1 – Grade B; JIS K 0557:1998 (confirmed 2017) – Water used for industrial and wastewater analysis – A4 water.
Selecting a Water Source for Silica-Sensitive Applications
For silica-sensitive applications, it is important to select a high-quality water source as silica can impact the quality of the results as well as instrument performance and functioning. Milli-Q® IQ systems fitted with a 0.22 µm final filter are validated to purify and deliver water meeting or exceeding standards and norms that are usually considered in silica-sensitive applications. The systems include a precise combination of purification technologies to produce pure (data not shown) and ultrapure water (Figure 4 and Figure 5) respecting norms and standards in terms of silica levels (Table 4 and Table 5).
- Pretreatment: The pretreatment cartridge eliminates most contaminants (colloids including silica, particles, free chlorine, and minerals) using a combination of pleated filter and carbon block components to protect the RO membrane.
- Purification: The RO/EDI technology sequence demonstrates good silica removal over a continuous period without need for resin regeneration.
- Storage: Water quality during storage is maintained thanks to the tank’s design which includes UV radiation and a specific vent filter. Stored pure water can be safely used depending on the application’s water quality requirements.
- Polishing: Water quality can be further improved to obtain ultrapure water by the addition of polishing cartridges containing IEX resins, which remove remaining traces of silica and other ions.
Milli-Q® IQ systems deliver ultrapure water with low and stable levels of silica that can be used for highly critical applications. Moreover, despite the lack of correlation between silica levels and resistivity values, the systems accurately measure water resistivity inline and display the value on the interface at the point-of-dispense as an indicator of system global performance. Additional design features on the most recent Milli-Q® IQ water purification systems offer time, accuracy, and precision benefits to users, such as the easy flow rate control and dispensing flexibility. Benefits such as ease of use, convenience and data traceability can lead to greater laboratory efficiency and simplified workflows for scientists.

The Importance of Water Quality for Silica-Sensitive Applications
High-quality water is needed to obtain reliable and reproducible results for silica-sensitive applications as silica can interfere with analysis accuracy and instrument performance. The combination of RO and EDI water purification technologies yields water of consistent and reliable quality suitable such critical applications. Pure water delivered by Milli-Q® IQ 7 series systems can be used for sensitive applications, while ultrapure water, obtained with additional polishing steps such as IEX resins leading to very stable silica content values, can be used for more critical applications (e.g., microelectronics). Moreover, water purification systems with inline quality monitoring and data management tools ensure data reliability and traceability, which is a prominent asset for laboratories working in standardized environments.
Contact us if you would like professional assistance in selecting a water purification system for your laboratory.
Acknowledgements
Thanks to the analytical laboratories of Merck’s R&D Lab Water Solutions facility in Guyancourt, France.
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?