Building Regional Single-Use Supply Chains
Regional Manufacturing to Mitigate Global Disruptions
To support the continued growth of biopharma manufacturing, you need a consistent, secure supply of single-use technologies. Given the potential for disruptions, managing supply chain risk is essential. That’s why we reimagined our supply chains for single-use technology manufacturing, investing in regionalized manufacturing supported by a global network.
Regional Single-Use Manufacturing within a Global Network
In our new global-local hybrid model, global oversight meets regionalized manufacturing to provide supply resilience and reliability. We’re strengthening our agility and improving flexibility by increasing our in-region, for region production capabilities across the globe. In addition to our existing site in Danvers, USA, we're expanding our sites in Molsheim, France, Wuxi, China and building a new facility in Daejeon, South Korea.
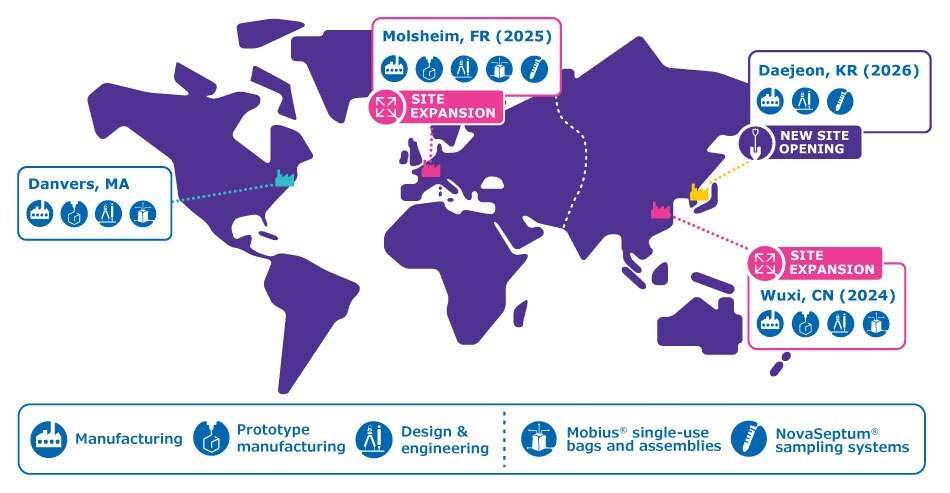
Regional manufacturing sites for single-use solutionsA world map showing manufacturing locations for single-use solutions in Danvers, USA, Molsheim, France, Wuxi, China, and Daejeon, South Korea.
We expanded our footprint for single-use technology manufacturing by investing:
- 130 € million in our Molsheim, France manufacturing facility, increasing capacity for bags and single-use assemblies by 150%
- 100 € million over 6 years to expand the existing Wuxi, China site, significantly increasing single-use assemblies and custom design capabilities in the region
By the end of 2025, we will have invested 280 € million in total and will have expanded our global single-use manufacturing network capacity fourfold and increased headcount by 2,550.
Each site will handle all aspects of single-use design, prototyping, engineering, and production, and by 2027, all our manufacturing sites will have the same comprehensive production capabilities. This will enable fully independent regional manufacturing, and 100% global product redundancy.
All sites operate under a Global Validation Master Plan, with ISO 9001 and ISO 14001 certifications, and are built for redundancy. If a disruption impacts your local site, our global network is equipped to deliver consistent single-use technologies, manufactured with equivalent quality standards, processes and equipment, to ensure your business continuity.
This means that no matter where you are globally, you can expect high-quality single-use technologies adhering to global quality standards, made locally, including:
- Mobius® 2D and 3D Assemblies
- Lynx® Connectors
- Mobius® Single-Use Mixers
- Mobius® Single-use Bioreactors
- NovaSeptum® Sampling Solutions
- Single-use Filling Assemblies
Strategic Raw Material Management
By expanding existing sites and creating new manufacturing sites, we’re providing critical supply redundancy for our customers. At the same time, we are going a step further, reducing risk in our raw material supply chains.
- We’re partnering with current suppliers to diversify and regionalize raw materials sourcing
- Each existing site (Danvers, Molsheim, Wuxi) is qualifying additional local raw material suppliers to enhance resilience through dual sourcing, and is increasing safety stocks to improve supply robustness
- We are analyzing supply chains to minimize overlapping dependencies for manufacturing our single-use technologies
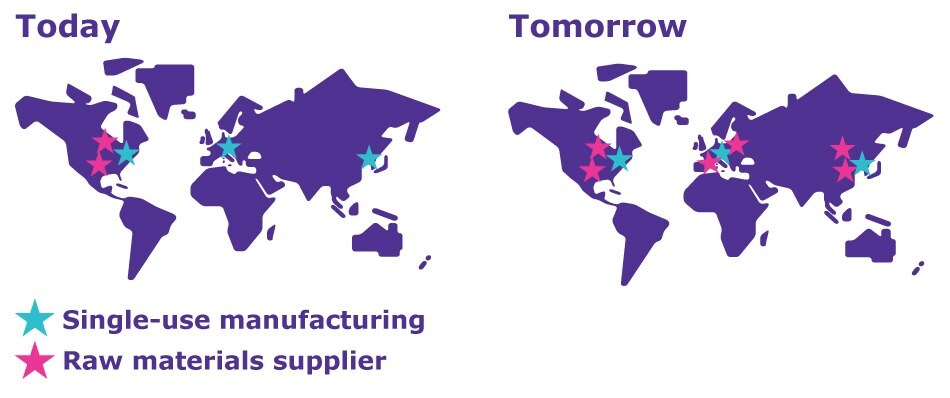
Current and future locations of single-use manufacturing sites and raw material suppliers, showing the increasing diversification and regionalization of our raw material supply.
Robust Quality Systems for Product Reliability
Our quality systems and optimized operational procedures ensure consistent and reliable production of high-quality single-use technologies through robust management systems that include ISO 9001 (quality) and ISO 14001 (environmental) certification.
In response to industry-wide challenges, we have implemented a three-pillar strategy to improve the quality of single-use technologies through reducing particulates and leaks and minimizing operator errors during handling and use of single-use assemblies at our site. We achieve this through:
- Raw material, environmental, and manufacturing cleanliness and quality controls
- Quality by design: component and assembly qualification and testing
- Quality risk management: sterilization and in-process, release, packaging, transport, and point-of-use testing
- Handling and operator training for best practices at our facility
Implementing single-use technologies can be complex but our Validation Services team can help. With deep knowledge of biopharma processing and regulatory requirements for global markets, we can help you design and apply the right validation strategy for smooth implementation and use of your single-use system.
Emprove® Dossiers are available for a range of products, and provide comprehensive documentation to facilitate your qualification, risk assessment, and process optimization. We’re also investing in supply chain digitization through our eMERGE™ program, giving you access to harmonized quality supplier data, predictive analytics, and faster issue resolution.
We know that change control is a crucial aspect of any biomanufacturing operation. Through our comprehensive, transparent change notification program we control, manage, and communicate changes to ensure you have no surprises and maintain uninterrupted supply of key technologies for your manufacturing.
Sustainability Built In
Sustainability isn’t an extra—it’s embedded in how we build and operate:
- Working with local raw materials suppliers and local customers to reduce shipping distances and transportation-related carbon emissions
- Embedding sustainability into our product development processes to take a holistic approach across the product lifecycle, from R&D to manufacturing, logistics, packaging, and recycling
- Working towards energy and resource efficiency at our sites globally
- The new production and warehouse building in Wuxi has been awarded the LEED Silver certificate, and the administration building achieved Platinum status
- We signed virtual power purchase agreements for European operations and have used 100% renewable electricity in USA since 2022. In China, about 40 % of electricity is covered by a solar power purchase agreement (PPA).
- In 2025, we signed a 20-year Power Purchase Agreement in South Korea to add 16 MW of renewable electricity capacity starting in December 2027, which is expected to serve approximately 75% of our Life Science business electricity demand in South Korea.
Related Products
如要继续阅读,请登录或创建帐户。
暂无帐户?