Bioprocess Considerations for Manufacturing Allogeneic Cell Therapies
- Comparing the Allogeneic and Autologous Cell Therapy Development Process
- Unique Needs of Emerging Biotechs Advancing Allogeneic Therapies
- Bioprocess Considerations for Allogeneic Cell Therapy Manufacturing
- Sterility Assurance Considerations for Allogeneic Cell Therapy Manufacturing
- Case Study: Media Production
- Set the Stage for a Successful Allogeneic Cell Therapy Tech Transfer
- Related Products
- References
Cell therapy represents a promising modality for the treatment of many diseases for which small molecules and biologics have not been successful in treating. As of December 2023, there have been 33 cell and gene therapies approved by the United States Food and Drug Administration (FDA)1 and thousands of candidates in clinical development.2 Significant investment continues to support growth of this segment and is expected to reach USD 5,650.3 million by 2025.3
Comparing the Allogeneic and Autologous Cell Therapy Development Process
There are many different cell types used for allogeneic cell therapy and depending on the culture platform that one chooses (based on the technology available and cost), the manufacturing process is not templated. The manufacturing process is typically performed at a larger scale and requires more complex manipulations compared to autologous manufacturing processes. Hence, there are stricter manufacturing requirements. In addition, there are other significant challenges that include process development, labor, logistics, hardware, and space.
Unique Needs of Emerging Biotechs Advancing Allogeneic Therapies
Companies advancing cell and gene therapies include both established, global biopharmaceutical companies and emerging biotechs. In many cases, early-stage organizations partner with a contract development and manufacturing organization (CDMO) due to a combination of limited funds, the high costs to set up and run a GMP facility, and the need for a high level of expertise to run the GMP manufacturing site.
To help ensure a smooth and efficient technology transfer to a CDMO, emerging biotechs should be mindful of the considerations related to the bioprocess and sterility assurance described below.
Bioprocess Considerations for Allogeneic Cell Therapy Manufacturing
Establishing a Robust Process
Regardless of the culture platform used to produce an allogeneic cell therapy, the process must be robust; critical parameters such as cell quantity and quality must be consistently met at each stage of the manufacturing process. One way to achieve this is to leverage quality by design (QbD) principles to establish the manufacturing process. QbD combines scientific knowledge such as the molecular and cellular characteristics of the final cell product with a risk analysis of process parameters such as oxygen concentration and incubation times and their effect on the quality of cells. It is also essential to ensure the quality of raw materials to support a reproducible and robust production process. One may want to reach out to vendors and secure batches of raw materials (especially for complex materials like fetal bovine serum) to ensure consistent production across the production campaign.
Ultimately, if the process is not robust, out-of-specification events may occur. This could lead to early termination of batches, delay of product release, necessitate time- and labor-intensive investigations, and may require repeat production.
Process Compatibility with GMP Suites and Conditions
Another critical consideration is the need for the process to be compatible with the GMP suite conditions. For example, the use of any equipment that increases the humidity in the GMP suite should be avoided. Water-containing equipment is conducive to the growth of microorganisms such as mold and fungi and increases the contamination risk of the final product. Alternative systems and equipment, such as dry baths and non-humidified incubators, can be used. However, the manufacturing process must be validated for these systems.
Sampling Plan
A sampling plan is essential to the manufacturing process. Samples for testing purposes are collected at multiple points along the allogeneic cell therapy manufacturing process. The samples are tested for endotoxin, cell numbers, cell quality, and functionality.
Depending on the availability and complexity, testing can be performed in-house or outsourced and should be established prior to the technology transfer. In addition to considering where the tests will be conducted, how and when the samples will be shipped must be determined to ensure sample integrity which is critical to the final test results. The collection method must also be established and include the volume, the containers to be used, and preferably collected in a closed-process manner.
Media Production and Management
Media and buffers (collectively referred to as media here) are critical components in the manufacturing processes; media production planning is, therefore, essential to ensure a sufficient supply throughout the entire manufacturing workflow. A key consideration related to media is whether to produce it in-house or outsource it from validated and reputed vendors. This decision generally is guided by the complexity of the media, process volume requirements, shipping costs, the cadence at which media are required, storage requirements, and shelf life/stability. Media also need to be tested for bioburden and endotoxins and the time required from testing to release must be taken into account to ensure that there are media for the cell production process.
A series of studies are also needed to ensure reproducibility of media formulations and include a determination of solubility and shelf life/stability. Media specifications such as pH and osmolarity must also be defined.
Sterility Assurance Considerations for Allogeneic Cell Therapy Manufacturing
GMP Grade Materials
Sterility assurance is also a critical part of GMP and begins in process development by ensuring that raw materials are suitable for GMP manufacturing. When it comes time to execute a tech transfer to the contract manufacturer, pharma- or IPEC-grade materials will be required, otherwise, it may be necessary to source and validate the proper materials, delaying the overall manufacturing timeline. In terms of consumables such as pipettes and single-use assemblies, they must meet a stringent sterility assurance level (SAL) of 10-6. Also, these consumables should be double, or triple wrapped in plastic with no paper backing which may release undesirable particles in the suites when the packaging is opened.
Reagents must be qualified for human use, be free of contamination and adventitious agents, and if possible, should be of non-animal origin. If animal components cannot be avoided, a risk assessment of these components must be performed, and based on the outcome of the risk assessment, appropriate control strategies implemented. Reagents with a higher risk of contamination from mycoplasma such as fetal bovine serum, for example, should be triple filtered through a 0.1 µm filter.
Closed Processing
Because final cell therapy products cannot be terminally sterilized or sterile filtered, the entire process must be kept sterile at all times, and as such, closed processing is preferred to minimize the risk of contamination. Sterile connections or welding/sealing can be used to connect or disconnect separate fluid paths between unit operations and maintain the sterility of the process path, even in lower classified environments.
When open processing cannot be avoided, manipulations such as the transfer of media or cells should be minimized, as each additional manipulation is a contamination risk. Alternative solutions should also be explored. For example, instead of cryovials, some vendors offer cryobags which may be aseptically connected to cell processing systems post cell-thawing, all in a closed manner. Similarly, the need to transfer growth factors from several small vials to the bulk media can increase the risk of contamination due to the multiple open-process manipulations. A possible solution is to ask your growth factor supplier to provide these media supplements in customized media bags of larger volumes.
Overall, a risk assessment of contamination from open processes should be conducted and the sterility of the process can be tested by aseptic process simulation (APS) which is described below.
Sterile Filtration
When liquid reagents are to be sterile filtered, the filters should undergo pre-use post-sterilization integrity testing (PUPSIT) or the solution should preferably undergo double filtration. For PUPSIT, the sterilizing filter and assembly must be tested for integrity before it is used for sterile filtration. In double filtration, two filters are used and pressure monitoring across the filters must be in place to ensure that the differential pressure across the filters is not exceeded. In both cases, the pre-sterilizing filtration burden must be less than 10 CFU per 100 mL. After using the filters in the process, the filters must undergo integrity testing to ensure that the filter integrity is not affected during the filtration process. Integrity testing is also applicable to vent filters and sterile air filters.
Extractable and Leachables (E&L)
The possible presence of E&L from the product contact part needs to be assessed for the risk of specific interactions between the raw materials and the cells. Ideally, E&L should be inert and do not affect the quality of the cells. When using single-use components that consist of additives or antioxidants, a risk-based approach should be considered.
Particulates
Particulates can be a potential health risk and may be present in the process path. The potential sources of these particulates include consumables such as pipettes, single-use assemblies, media bags and connecting tubes. While particulates in the process path cannot be avoided, and sterile filtration of the final product is not possible, there are steps that can be taken to minimize their presence in the process. These steps include visual checks of consumables prior to use, incorporation of particulate filters with a pore size between 40 and 100 µm before the final fill stage, and visual inspection of the product by trained operators post-final fill. Rinse studies should also be carried out and particle libraries created to curate the particles that are found in the process. Any particles that are found should be characterized to determine whether they are intrinsic in the process and if they are potentially hazardous to the patients.
Aseptic Process Simulation (APS)
APS is a key component of sterility assurance. Because cell therapy manufacturing does not allow the final cell product to be sterile-filtered, sterility must be maintained throughout the entire process. It is evaluated by simulating the process with tryptic soy broth, a media that supports the growth of micro-organisms, to challenge the:
- Sterility of the process
- Operators executing the process steps
- Environment in which the processes are performed
When the APS is carried out for the first time, three consecutive successful runs must be executed; subsequent simulation is to be performed once every six months. In most cases, the APS is designed and performed by the CDMO and includes the most complex aseptic processing steps. It must include 50% of closed processes and 100% of the open processes. APS is a requirement for GMP manufacturing and should be factored into overall timelines and budgets.
Case Study: Media Production
The following case study uses media production to highlight the bioprocess, and sterility assurance considerations described above. In this scenario, 150 liters of media is needed for the manufacturing bioprocess, and to be filled into ten 15 L bags (Figure 1).
From a process perspective, it is essential to know what media will be produced, the required raw materials and amount to be used, and solubility data which includes how long it takes to dissolve the media powder, at what temperature, and at what mixing speed. There must also be plans to make excess media (in this example, 200 L of media is prepared, an excess of 50 L) for safety stock, for testing, and to account for lost media trapped along the process line. Media specifications including pH and osmolality must be defined. For sterile filtration, there must be a sample collection for the pre-filtration bioburden testing.
Twelve media bags are filled; ten bags will meet the processing requirements, one will be used as a safety stock, and one used for post-filtration QC testing. Shelf life and stability of the media produced must also be established.
In terms of sterility assurance, all materials used in the process should be suitable for GMP manufacturing. E&L of the single-use assemblies should not have detrimental effects on the cells, and materials and consumables should be visually checked for particulates before use. Double sterile filtration is used with the control measures, and the pre-filtration bioburden must be less than 10 CFU per 100 mL. Finally, the media fill process will be completed using closed processing. As noted above, one bag of medium will be used for QC testing for bioburden and endotoxin. Before the start of the first media production, APS will be performed three consecutive times, after the sterile boundary or post sterile filtration; following this time period, APS will be performed every six months. As a close system is used, the APS will simulate 50% of the media fill.
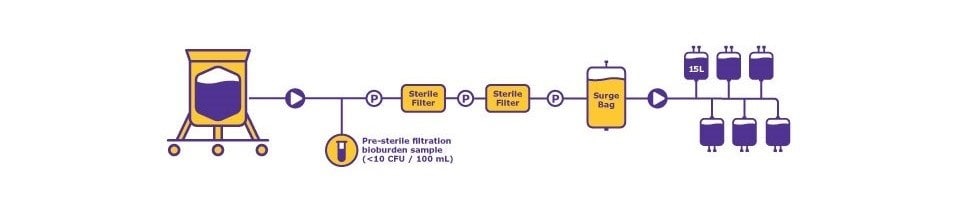
Figure 1.Process requirement = 150 L (10 x 15 L media bags). Media production scenario highlighting key process, production, and sterility assurance considerations. “P” meaning Pressure Sensor.
Set the Stage for a Successful Allogeneic Cell Therapy Tech Transfer
A successful allogeneic cell therapy workflow begins with the development of a GMP-compliant process. The process and raw materials must be locked, along with documents such as working instructions and batch records, in preparation for a smooth and efficient technology transfer to your contract manufacturer. Attention to the bioprocess and sterility assurance considerations described above will help set the stage for a successful transfer.
We provide information and advice to our customers to the best of our knowledge and ability but without obligation or liability. Existing laws and regulations are to be observed in all cases by our customers. This also applies in respect to any rights of third parties. Our information and advice do not relieve our customers of their own responsibility for checking the suitability of our products for the envisaged purpose.
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?