Determination of Sinomenine Hydrochloride in Zhengqing Fengtongning Tablets
Abstract
A method for the determination of sinomenine in Zhengqing Fengtongning tablets, following one public draft of the monograph method for Zhengqing Fengtongning tablets proposed by the Chinese Pharmacopeia, was developed. After sample preparation, using solvatization in ethanol and filtration, sinomenine was determined by HPLC-UV using a Discovery® C18 column. Results showed the limit of detection (LOD) and limit of quantification (LOQ) for sinomenine in the calibration range from 2 to 800 μg/mL were 125.7 and 380.8 μg/g tablet sample.
Section Overview
Introduction
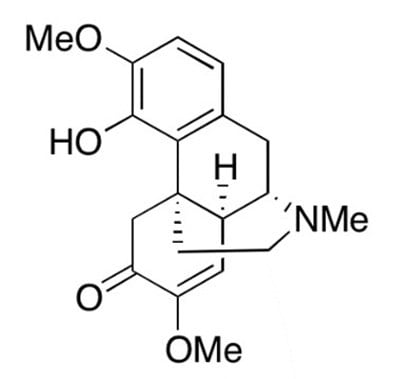
Figure 1.Chemical structure of sinomenine.
Sinomenine (Figure 1), a bioactive alkaloid, is an active ingredient extracted from the traditional Chinese medicinal plant Qinfengteng.1 In vivo and in vitro experiments have confirmed that sinomenine has biological effects such as anti-inflammatory, antioxidant, inhibition of cell apoptosis, and immunosuppression.2 Zhengqing Fengtongning tablets are derivatives related to sinomenine, which have been approved by the National Medical Products Administration of China and the National Health Insurance Directory for the treatment of rheumatoid arthritis (RA). Huang et al.3 and Liu et al.4 confirmed that Zhengqing Fengtongning tablets can inhibit the progression of RA inflammation by regulating the secretion levels of inflammatory factors and macrophage subpopulations by utilizing collagen-induced arthritis mouse models and peripheral blood from RA patients.
In 2024, the official website of the Chinese Pharmacopoeia Commission released a draft of a detection method for sinomenine hydrochloride in Zhengqing Fengtongning tablets.5 Once it passes method validation, this method will be included in the 2025 edition of the Chinese Pharmacopoeia. Accordingly, this work describes the development and evaluation of a reversed-phase HPLC method using a Supelco® Discovery® C18 column for the quantification of sinomenine in a commercially available brand of Zhengqing Fengtongning tablet.
Experimental
Standards and samples were prepared and analyzed according to the following procedures:
Standard Preparation
- Stock solution: Weigh 10 mg of a sinomenine reference standard into a 10 mL amber glass volumetric flask. Add approximately 3 mL of ethanol and sonicate for 5 minutes. Top up to the mark with the mobile phase and mix well. The concentration of sinomenine in the resulting stock solution is 1 mg/mL.
- Standard solutions for external calibration: Separately pipette 2, 4, 8, 20, 40, 80, 160, 400, and 800 µL of the stock solution into 1 mL vials, and fill up to 1 mL with mobile phase to obtain a series of standard solutions with sinomenine concentrations of 2, 4, 8, 20, 40, 80, 160, 400, and 800 µg/mL.
Sample Preparation
Weigh ten tablets of Zhengqing Fengtongning (~ 1.6 g) precisely to 0.1 mg and grind to powder. Transfer ~ 0.16 g of powder into a 100 mL volumetric flask to get the equivalent quantity of 20 mg sinomenine hydrochloride (the label indicated a concentration of 20 mg/tablet). Add 30 mL of ethanol and 0.1 mL of ammonia solution (400 mL of concentrated ammonia, diluted with water to a total volume of 1000 mL). Sonicate the mixed solution for 20 minutes at 300 W and 25 kHz. Cool to room temperature, then add mobile phase to the mark and shake well. Finally, filter through a 0.45 μm membrane before HPLC analysis.
HPLC UV Method
For the HPLC-UV analysis, a Discovery® C18 column was used (Table 1).
Acceptance Criteria for Standard Solution
Theoretical plate number calculated from the peak of sinomenine: NLT 2,000.
Results & Discussion
Zhengqing fengtongning tablets were ground into a powder and then extracted with ethanol. The resulting solution was then filtered and analyzed for sinomenine on a Discovery® reversed-phase C18 HPLC column using an external calibration. Then the content of sinomenine hydrochloride in the tablet samples was calculated by multiplying the content of sinomenine by 1.219.
Chromatographic results for the HPLC analysis of the sinomenine standard and blank solution are displayed in Figures 2 & 3. The developed method showed a plate count of 12,601 and therefore meets the criteria of NLT 2,000 (Table 3). The eluting peak was very symmetrical with a tailing factor of 1.1.
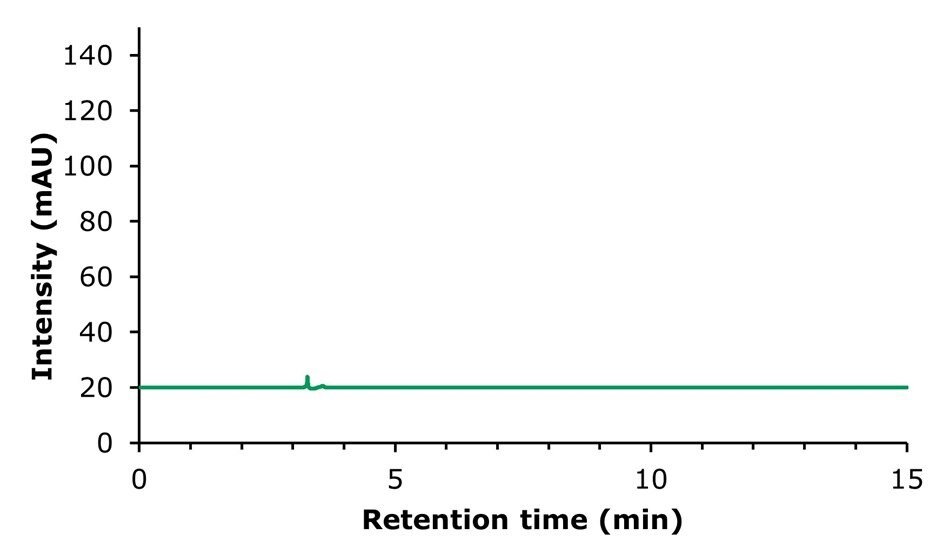
Figure 2.Analysis of a blank solution (mobile phase: [A] acetonitrile; [B] 0.78% sodium dihydrogen phosphate solution, A:B 12:88).
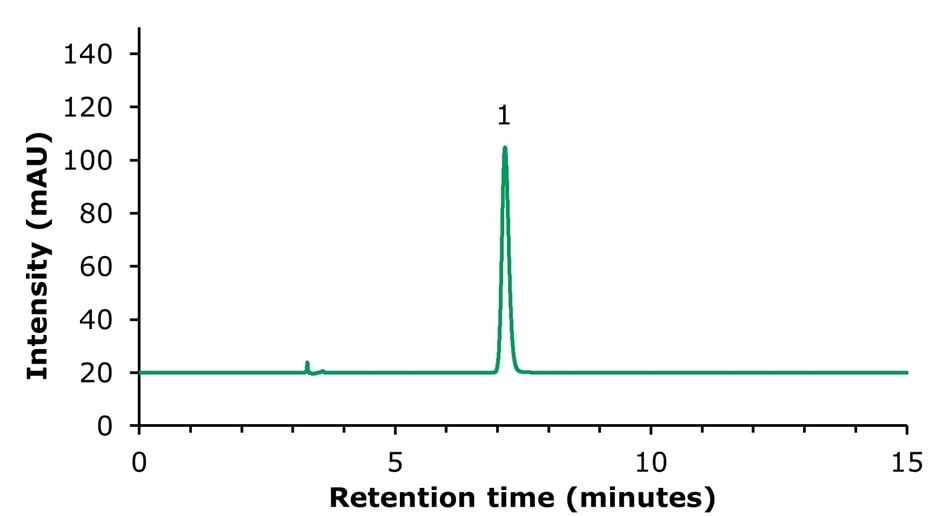
Figure 3.Standard solution containing 160 µg/mL of sinomenine (1).
Calibration and Repeatability
The calibration curve for sinomenine is shown in Figure 4. The calibration in the range of 2 to 800 µg/mL showed a linearity value R2 of 1.000. The method also showed excellent repeatability with an RSD value of 0.07% for the 160 µg/mL standard solution (Table 2).
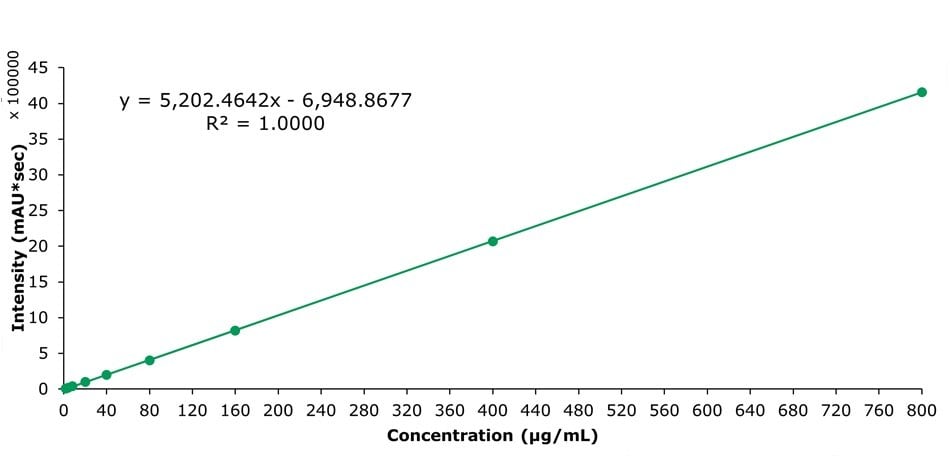
Figure 4.Calibration curve for sinomenine obtained by the analysis of nine calibration solutions.
Method Sensitivity
From the calibration range of sinomenine (2 to 800 μg/mL), the low concentration standards were used for sensitivity determination. The obtained results for the derived limit of detection (LOD) and limit of quantification (LOQ) for sinomenine hydrochloride dihydrate in the tablet sample were 125.7 and 380.8 μg/g.
Sample Analysis
The analysis of a sample solution representing a tablet with 20 mg sinomenine hydrochloride in 100 mL (Figure 4) showed a content of 17.85 mg/tablet (Table 5).
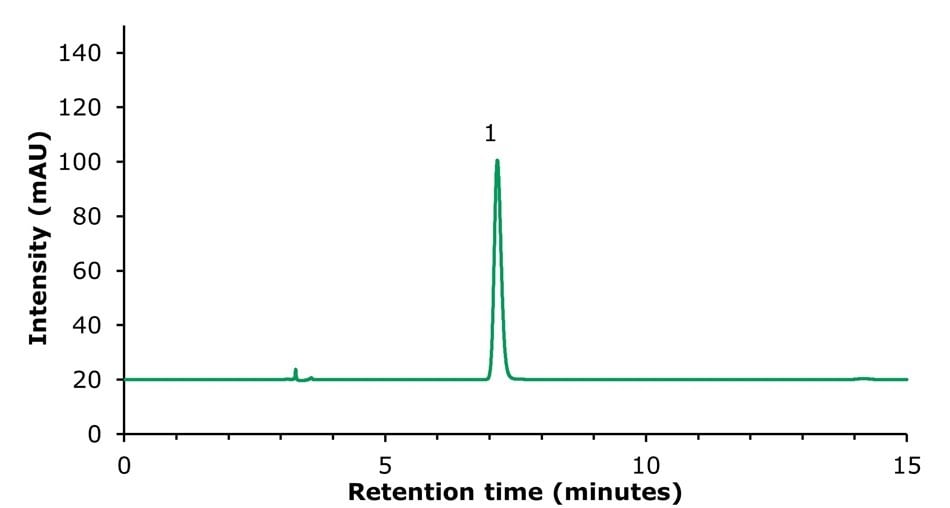
Figure 5.Sample solution for determination of sinomenine (1).
Conclusion
An HPLC-UV method was developed for the quantification of sinomenine in Zhengqing Fengtongning tablets, in accordance with the draft monograph published by the Chinese Pharmacopoeia. Sample preparation involved ethanolic extraction of ground tablet material, followed by chromatographic analysis on a Discovery® C18 column.
The developed method yielded a theoretical plate count of 12,601 (USP) for the sinomenine peak, thereby meeting the Chinese Pharmacopoeia draft monograph requirement of not less than 2,000 plates. The determined RSD for the method complied with general requirements of Chromatographic Method 0512 of the Chinese Pharmacopoeia, which stipulates an RSD of less than 2%, unless otherwise specified. This further underlines its applicability in the analysis of the active ingredient sinomenine in Zhengqing Fengtongning.
The calculated limits of detection (LOD) and quantification (LOQ) for sinomenine hydrochloride were 125.7 μg/g and 380.8 μg/g of tablet sample, respectively. These results confirm the suitability of the method for routine quality control and quantification of sinomenine in Zhengqing Fengtongning tablets.
Reference Standards
References
如要继续阅读,请登录或创建帐户。
暂无帐户?