Formulation Strategies for mRNA Vaccines and Therapeutics
mRNA technology has proven its value as a vaccine and is increasingly being explored for various medicinal applications for cancer therapies, genetic disorders, and autoimmune diseases, among others. Another technical article discusses making and purifying mRNA and the considerations and challenges in scaling up from process development to GMP manufacturing. This technical article will focus on formulating mRNA drug products for efficient delivery to patients.
View all of our products and services for mRNA development and manufacturing
Section Overview
Considerations for Mrna Manufacturing
mRNA vaccines and therapeutics are typically manufactured following a templated process (Figure 1). This template can be adapted to any mRNA target with minimal process and formulation changes enabling mRNA manufacturers to quickly switch to new target molecules.
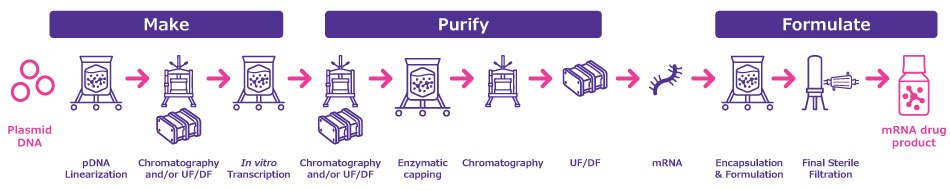
Figure 1.General process template for mRNA manufacturing.
Several critical factors significantly influence the process, yield, and quality of the final mRNA product. During in vitro transcription and subsequent purification steps, the mRNA remains vulnerable to degradation. The risk of enzymatic degradation can be minimized by using endonuclease activity-free, high-quality chemicals. Consequently, careful selection of raw materials and process chemicals is key to reducing RNase-induced degradation and enhancing mRNA stability throughout purification and formulation.
This webpage will provide a roadmap for efficient processes and summarize advanced strategies for manufacturing stable and effective mRNA drug products. Our brochure “Process chemicals for mRNA drug manufacturing" provides the details you need to make informed choices."
Formulating the mRNA
Delivery tools are crucial to ensure effectiveness of mRNA vaccines and therapeutics which is why the different delivery mechanisms should be considered prior to formulation (Figure 2).
The most advanced delivery approaches are based on combinations of lipids and polymers that bind to the mRNA. Lipoplexes are complexes of cationic lipids that bind to and protect the nucleic acid and facilitate its transport into the cell by fusing with the cell membrane. Polyplexes are highly stable complexes of positively charged polymers, such as polyethyleneimine (PEI), and negatively charged nucleic acids. These complexes efficiently deliver mRNA to cells, can have tailored release profiles, and are well-suited to therapeutic applications.
Lipid nanoparticles (LNP) comprise cationic and helper lipids that form vesicles to protect the mRNA. This commonly used mRNA delivery platform is ideal for rapid delivery and high expression levels and is the focus of this technical article.
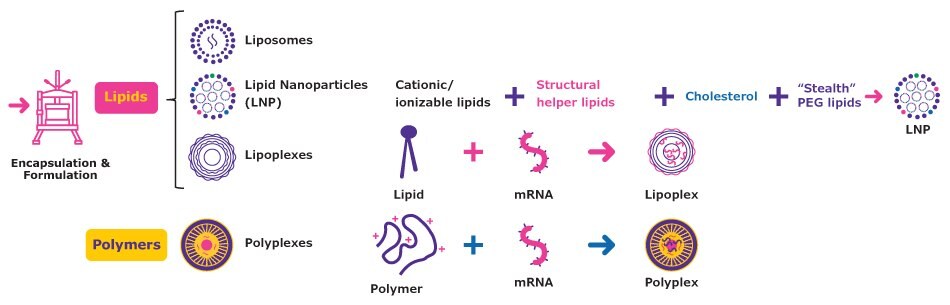
Figure 2.Commonly used mRNA delivery systems.
Considerations for Lipid Selection
Lipid selection and lipid combinations for formulation of mRNA drug product is a rapidly evolving field with significant implications for future development of mRNA therapies and vaccines. When choosing lipids, it is essential to consider the delivery route of the mRNA drug product to ensure maximum effectiveness and optimal biodistribution; each delivery route has its own requirements for lipid size, stability and composition.
Along with lipid selection, the ratio between individual lipids is crucial for fine-tuning characteristics and directly impacts bilayer fluidity and LNP fusogenicity. When choosing a lipid, multiple factors should be considered, including type, source, and quality, which directly influences impurity profile and properties such as particle characteristics, stability, and release profile in the final formulation. Consistent lipid quality is necessary for reproducible performance and is heavily reliant on the raw materials' quality used for lipid synthesis and the lipid's material characteristics. Synthetic lipids with a consistent, high-product quality coupled with tailored support and services by a trusted partner are ideal to meet individual needs and ensure an optimal final product performance. Each LNP consists of four different lipids which envelop the mRNA target, protecting it from degradation:
- Cationic/ionizable lipids encapsulate the mRNA via electrostatic interactions. Delivery to hepatocytes (for boosting or silencing of protein expression) requires ionizable lipids (passive targeting, endosomal release) whereas uptake by immune cells is much easier. Strong cationic lipids also serve this purpose and are responsible for the efficient release of RNA into the cytoplasm. The structure of cationic lipids significantly affects LNP activity, toxicity, and biodistribution.
- Polyethylene glycol (PEG) lipids provide colloidal stability and prevent protein binding to the particle, thereby shielding it from the immune system and achieving longer circulation. The length of the PEG chain and fatty acid chains determine the circulation lifetime and fusogenicity, or how well the particle can fuse with the endosomal membrane of the LNP. For extended circulation, longer fatty acid chains, like polyethylene glycoldistearoylglycerol (DSG PEG 2000), can be utilized. PEG concentration also affects particle size. However, PEG use may lead to antibody formation, potentially compromising immunization effectiveness.
- Neutral/anionic lipids provide structural stability and play a role in defining the fusogenicity and biodistribution. 1,2-dioleoyl-sn-glycero-3- phosphoethanolamine. For example, it was shown that LNPs containing 1,2-dioleoyl-sn-glycero- 3-phosphoethanolamine (DOPE), which plays an important role in endosomal release, led to enhanced delivery of mRNA to the liver as compared to 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC).1 Study results suggest that these helper lipids also assist in the stable encapsulation of the RNA.2
- Cholesterol is used to modulate the bilayer density, fluidity, and uptake (raft formation) of the LNP. While there are animal-derived and synthetic versions of cholesterol available in the market, synthetic cholesterol offers several advantages, including higher purity, lack of animal-derived molecules such as prions, scalability, and highly consistent quality.
For further insights into mRNA formulation, read our white paper Considerations for Advancing a Lipid Nanoparticle Formulation to Clinical and Commercial Manufacturing .
LNP Formulation: Stability and Other Considerations
LNP Formulation
The purified mRNA can be formulated into the delivery particle via different methods, with solvent injection being the most common approach. Lipids are dissolved in a solvent, such as ethanol, and rapidly mixed with an aqueous, low pH buffer containing the mRNA using crossflow mixing or microfluidic mixing to create the LNPs. The low pH buffer is then dia filtered into a neutral buffer and ultrafiltration is used to concentrate the particles. It is crucial that this tangential flow filtration (TFF) step is conducted swiftly, as prolonged exposure to low pH can lead to lipid hydrolysis, leading to formation of impurities such as hydrolipids that may compromise the lipid bilayer structure, stability of the formulation and drug release characteristics. Degradation of the lipids can also increase the size of the particle, resulting in aggregation. The typical process flow is illustrated in Figure 3.
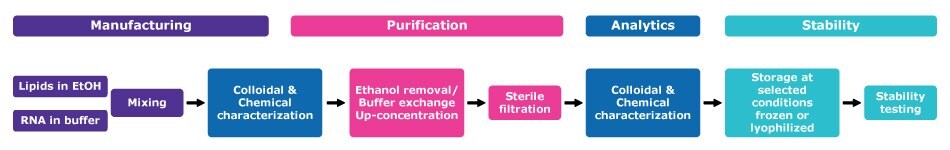
Figure 3.LNP manufacturing, purification, and stabilization process flow.
LNP Advantages and Challenges
LNPs have excellent stability, structural plasticity and enhanced gene delivery compared to other delivery systems. They increase the rate of transfection as compared to naked mRNA and enable intravenous injection without the risk of immediate degradation of the target mRNA by RNases in the bloodstream. If specific ligands are incorporated, they also enable active targeting. Despite these benefits, LNPs present challenges: they generally require cold chain logistics and sterile filtration is not always possible. As product sterility is a requirement for approval and licensure, in some cases, alternative sterilization methods such as gamma irradiation, heat sterilization, high-pressure sterilization or closed processing must be considered to assure drug product safety.
mRNA and LNP Stabilization
Ensuring the encapsulation of mRNA within LNPs is crucial for maintaining mRNA stability in the final formulation during both processing and storage. Liquid LNP formulations often lack sufficient stability under the standard vaccine storage conditions and require cold chain transportation at -80 °C to -60 °C. To enhance LNP stability, lyophilization is a valuable strategy. However, stability following lyophilization depends on selecting the optimal storage matrix, identifying appropriate excipients, and optimization of the lyophilization process.
Excipients for LNPs
Selecting excipients with the appropriate quality and purity levels is critical for formulating stable mRNA drug products. High-quality excipients not only enhance the stability and effectiveness of the formulation but also play a vital role in ensuring patient safety and regulatory compliance. Utilizing endonuclease-activity-free Emprove® Expert excipients can significantly minimize risks associated with potential aggregation or degradation of mRNA and support the overall integrity of the product.
Key excipients to consider include:
- Surfactants: Non-ionic surfactants, such as polysorbate 80 or poloxamer, can be used to enhance the dispersion of lipids and prevent aggregation of LNPs.
- Buffers: The choice of buffer system is essential for maintaining the pH and ionic strength of the formulation, which can affect mRNA stability and LNP formation.
- Stabilizers: Stabilizers such as trehalose and sucrose help stabilize both the mRNA during formulation and the mRNA-LNP complex during all processing steps prior to patient administration
- Bulking agents and stabilizers: Bulking agents like mannitol and sorbitol are often used with stabilizers to form a glassy matrix that reduces molecular mobility and prevents degradation in lyophilization processes. While stabilizers contribute to improved stability through high glass transition temperatures, bulking agents can act as plasticizers, potentially lowering the glass transition temperature of the formulation. Consequently, a careful selection of these excipients is essential to ensure the stability of lyophilized formulations.
- Cryoprotectants: For formulations that require freeze-drying, cryoprotectants such as mannitol or glycerol can be added to protect LNPs from damage during the freezing and thawing processes.
Conclusion
By carefully selecting and utilizing high-quality excipients, manufacturers can enhance the stability and efficacy of mRNA formulations. Identifying a supplier that provides high-quality products, along with comprehensive documentation and technical expertise, helps to streamline the path to market. This strategic approach not only ensures product quality but also facilitates the rapid development of reliable and effective mRNA vaccines and therapeutics.
For a comprehensive overview of our high-quality chemicals and excipients, including a broad portfolio of endonuclease-free products, access our brochure Process chemicals for mRNA drug manufacturing
For more information on how we can assist you in advancing your mRNA projects, please reach out to our team of experts.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?