Extraction and Evaluation of Withanolides in Ashwagandha Root
Abstract
Accurate characterization of Withania somnifera (ashwagandha) preparations requires reliable quantification of key withanolides, which can vary depending on plant material and formulation. In this work, seven withanolides were analyzed using an HPLC-UV method designed to achieve chromatographic resolution in complex plant matrices. Certified reference materials, raw materials and commercial samples were compared to evaluate method performance. The results showed agreement with values reported by independent laboratories, indicating suitability for routine analysis. This approach supports standardization efforts for ashwagandha products and provides a basis for assessing product quality and composition.
Section Overview
Introduction
Withania somnifera is more commonly known as ashwagandha but can also go by the names of winter cherry and Indian Ginseng. It is grown in India, the Middle East and parts of Africa and is most extensively used in Ayurveda for its medicinal and pharmacodynamic properties. Many therapeutic properties are found in the leaves and root of the plant. The plant is collected, dried and then ground to a powder, which is traditionally mixed with water, milk or ghee. As this medicinal herb has gained popularity in Western countries, it has also become available in capsules and tablets.
Ashwagandha has a variety of elements that contribute to its therapeutic properties, such as, steroidal lactones (withanolides and withaferins), alkaloids (isopelletierine and anaferine) and saponins (sitoindoside VII and VIII).3,4 The most biologically active are the withanolides, a naturally occurring steroid. We focus on seven chemical constituents of ashwagandha, withaferin A, withanolide A, withanolide B, withanone, 12-deoxywithastramonolide, withanoside IV and withanoside V (structures shown in Figure 1). Plant cultivation and plant parts play a major role in the concentration of withanolides. For instance, withanolide A has been found to treat neurodegenerative diseases and is heavily present in the leaves, whereas withaferin A shows anti-angiogenesis and anti-cancer properties and is primarily found in the root.1,2 Some of the components of withanolides are found to be less effective however the synergistic effects cannot be disregarded. There are at least 50 different chemical profiles known that have been used in over 200 formulations.4
As the benefits of ashwagandha are becoming more widely known, the market unfortunately also contains products where manufactures substitute with other materials, use non-authentic material, or use inadequate testing methods leading to inaccurately reported concentrations.2 To ensure that consumers are receiving reliable product it is imperative that proper testing is achieved. However, validated testing protocols and certified reference materials are limited. Ashwagandha authentication can be achieved by DNA PCR test and chemical analysis by HPTLC and HPLC-UV. Each of these techniques can become troublesome in the instance of mixed or unauthentic samples which can also lead to challenges during clinical studies.
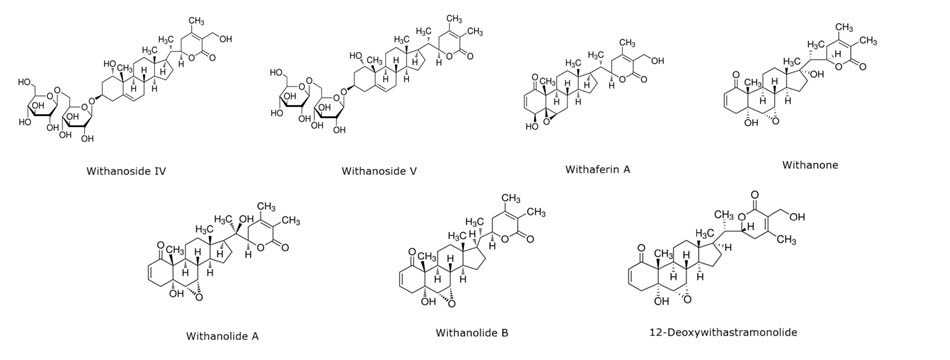
Figure 1.Seven major analytes present in ashwagandha root.
Experimental
NIST root powder and extract were obtained as part of the NIST Dietary Supplement Laboratory Quality Assurance Program (QAP) and were prepared as follows:
- Withanolides extracted from dried root powder: Accurately weigh 5 g of ground ashwagandha root powder and place in a 250 mL round bottom flask. Add 50 mL of methanol. Reflux at 65° C for 15 minutes. Decant into Erlenmeyer flask and repeat 5 times or until reflux is clear. Filter with 0.2 µm PTFE syringe filter. Evaporate using a Rotovap with a temperature setting of 35° C to approximately 40 mL. Transfer to a 50 mL volumetric flask and bring to volume with methanol.
- Withanolides reconstituted from dried extract: Accurately weigh 100 mg of dried ground ashwagandha extract and place into a 10 mL volumetric flask. Add 7 mL of methanol. Sonicate at 40° C for 20 min. Bring to volume with methanol and transfer to a centrifuge tube. Centrifuge at 4000 rpm for 2 minutes. Remove the supernatant and filter with 0.2 µm PTFE syringe filter.
Calibration Curve Preparation: Single point calibration curve was prepared by mixing 300 µL each of three certified reference materials (CRMs) in methanol (Table 1).
Samples were analyzed by HPLC-UV using the conditions listed in Table 2.
Results & Discussion
NIST root powder and extract obtained as part of the NIST Dietary Supplement Laboratory Quality Assurance Program (QAP) were prepared as described above and analyzed by HPLC-UV using the conditions listed in Table 2. Example chromatograms of a standard, root powder and extract samples are shown in Figures 2-4. The analysis of the seven selected analytes required careful integration as many matrix components elute very close to the analytes of interest.
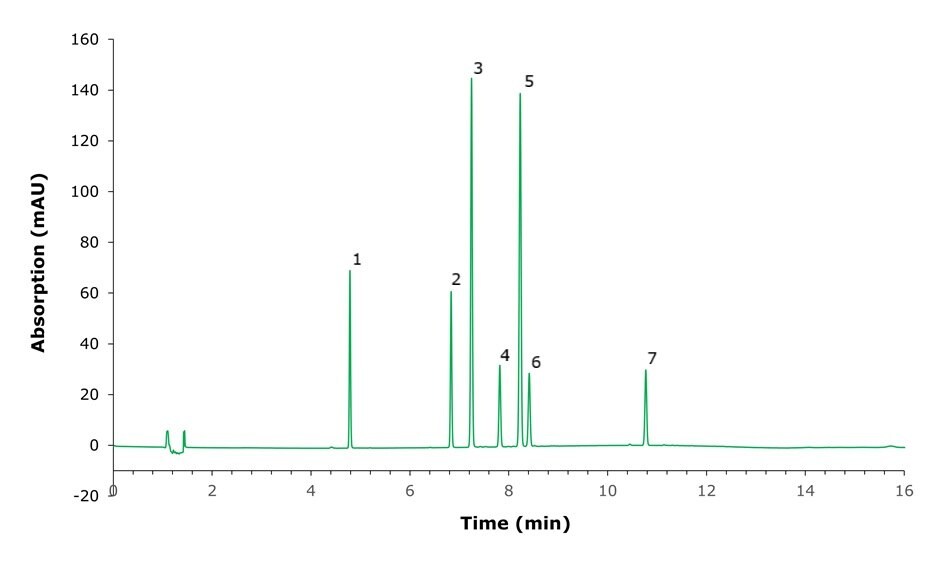
Figure 2.Chromatogram of certified reference materials A-197, A-204 and A-205, used for quantitation. Numbered peaks are identified in Table 3.
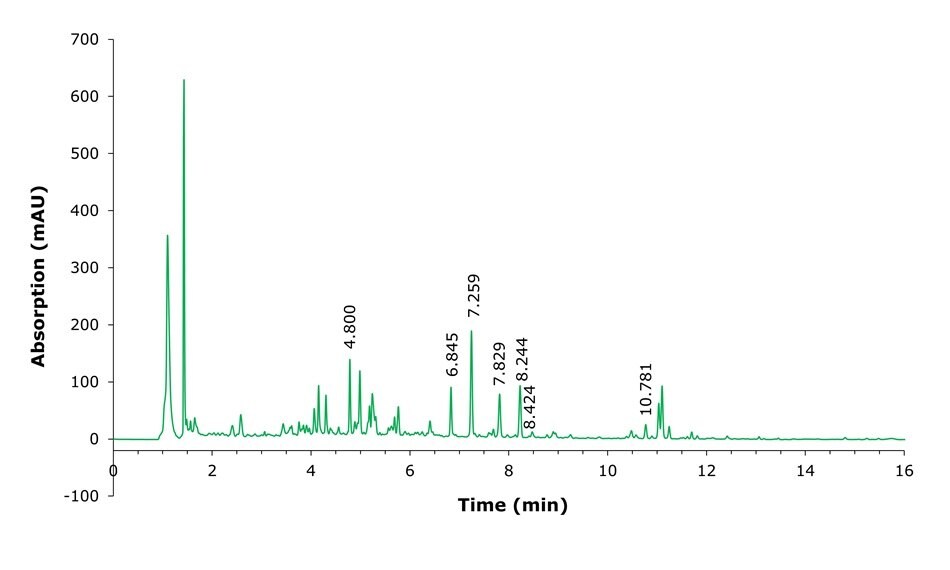
Figure 3.Chromatogram of NIST ashwagandha root powder.
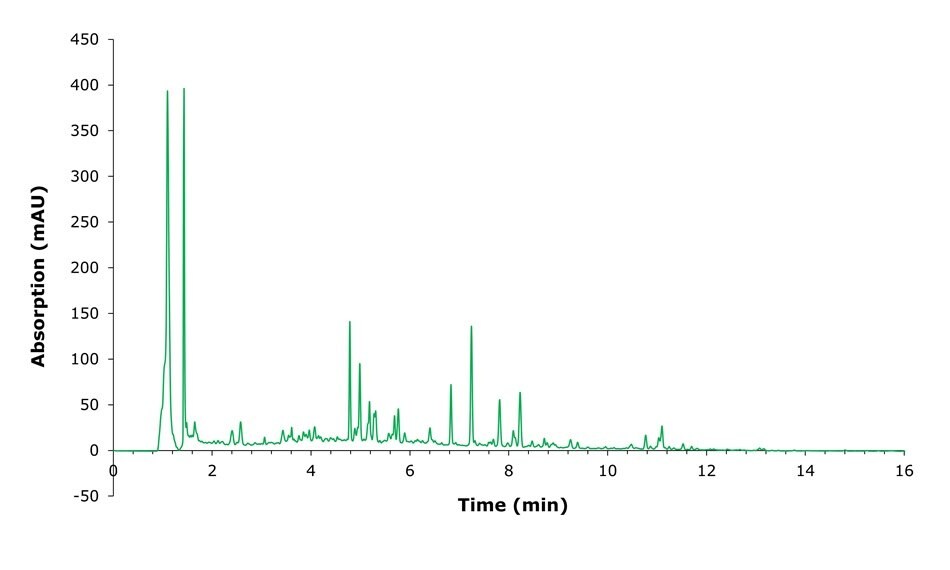
Figure 4.Chromatogram of NIST ashwagandha extract.
Quantitation was done by a single point calibration curve prepared on the same day as the sample extraction. The curve was fitted linear passing through the origin. The calibrant was injected in triplicate before and after extracted samples. The NIST samples were prepared in triplicate and injected three times each. Percent relative standard deviation of the bracketing calibrant injections (calibration solutions before and after plant samples) were below 1.5% for all analytes. Resolution of the two closest eluting analytes, withanolide A and withanone, was 2.6, for all other peaks resolution was >6 (Table 3).
From this data the percent composition of the analytes of interest were calculated using the formula in Equation 1 and results presented also in Table 3. Concentrations of withanolides determined from our analysis were within the consensus tolerance limits of the mean value from the participants of the QAP.
% w/w = (Concentration x Total Volume) / (Weight of Sample) × 100%
Equation 1. Percent composition by weight
Conclusion
An overview of the sample preparation and analysis for accurate quantification using a novel HPLC-UV method with the ability to resolve seven withanolides of interest is detailed. Matrix components of plant matrices can be very complex, as seen in the chromatograms of the root powder and extract samples, further emphasizing the need for reliable and robust methods. This study demonstrates that the provided method is in close agreement with the analyzed values obtained from other third-party laboratories. This method can aid in standardizing ashwagandha-based products while also ensuring their quality and potency. Overall, the analysis of withanolides in ashwagandha is critical for understanding the herbs therapeutic potential and guiding its appropriate use in traditional and modern medicine.
Solvents, Reagents & Reference Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?