Aniracetam Capsules Assay (HPLC) following Chinese Pharmacopeia 2015 Monograph Method on Ascentis® C18 with UV Detection
材料
相关产品
CONDITIONS
column
Ascentis® C18, 15 cm x 4.6 mm, 5 μm particles (581324-U)
mobile phase
[A} water; [B] acetonitrile, (65:35, A:B)
flow rate
1.0 mL/min
pressure
1305 psi (90 bar)
column temp.
25 °C
injection
10 μL
sample
SST Solution
说明
分析说明
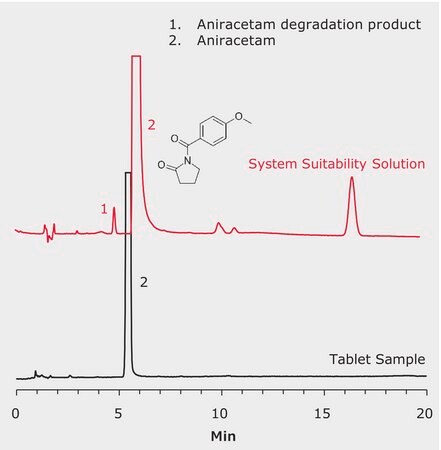
LC-UV Analysis of Aniracetam Capsules (Huilaxitan Jiaonang). Aniracetam, or N-anisoyl-2-pyrrolidinone, is a nootropic agent (substance that improve cognitive function). It is as a prescription drug world-wide, but not approved for use in the United States. The performance criteria per the Chinese pharmacopeia (ChP) 2015 monograph method are met. The detection limit (LOD) and limit of quantitation (LOQ) with HPLC-UV are both better than 1 ppm.
其他说明
SST Solution: Dissolve 50 mg of Aniracetam with 5 mL methanol into colorimeter tube, heat in water bath at 70 °C for 1h. Cool down to room temperature. Dilute with mobile phase to 1 mg/mL solution.
Standard solution: Dissolve appropriate amount of Aniracetam to mobile phase to obtain 80 μg/mL (80 ppm) standard solution.
Test solution: Take appropriate amount of drug powder from capsules into mobile phase to obtain 80 μg/mL (80 ppm) sample solution. Filter sample solution prior to injection through a 0.45 μm Millex PVDF filter.
法律信息
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany