LC/MS/MS Analysis of Warfarin in Plasma on Ascentis® Express C18 after SPE using HybridSPE®-Phospholipid
CONDITIONS
sample/matrix
rabbit plasma, unfiltered K2-EDTA, spiked with warfarin at 100 ng/mL (3:1, plasma:1% formic acid in acetonitrile)
SPE tube/cartridge
HybridSPE®-Phospholipid, 96-well plate (575656-U)
sample addition
to each well add 100 μL plasma, followed by a 300 μL of 1% formic acid in acetonitrile, agitate on orbital shaker for 4 minutes
elution
collect filtrate and anlyze directly
column
Ascentis® Express C18, 10 cm x 2.1 mm I.D., 2.7 μm particles (53823-U)
mobile phase
[A] 5mM ammonium formate, pH 4.2 with formic acid; [B] 5mM ammonium formate in 95:5 acetonitrile:water, 50:50 (A:B)
flow rate
0.3 mL/min
column temp.
35 °C
detector
ESI+, 100-1000 m/z
injection
2 μL
说明
分析说明
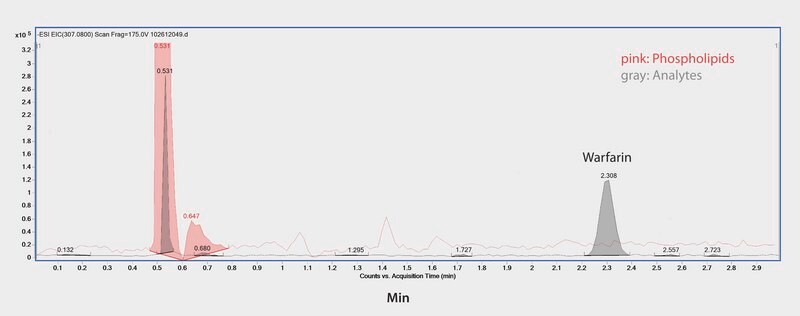
Warfarin is a widely prescribed oral anticoagulant for treatment and prevention of thrombosis and thromboembolism. Here we show the LC/MS/MS analysis of warfarin in plasma samples by reversed-phase chromatography with effective sample prep to remove endogenous phospholipids using HybridSPE-Phospholipid. UHPLC analysis utilized an Ascentis Express C18 column. The highest grade LC-MS solvents were used to supply low background interference and low particulate contaminants for robust, trouble-free operation. Cerilliant CRMs provided reliable identification and quantification.
法律信息
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany, HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany, null