MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A and Panel B: Next-Generation Cytokine Multiplex Assays
Cytokine multiplex assays allow researchers to easily investigate the immune system and inflammation mechanisms. Multiplex cytokine analysis saves time and resources by simultaneously analyzing multiple cytokines. The MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A and Panel B are multiplex cytokine panels that offer improved quality, workflows, and sample detection.
Section Overview
Role of Cytokines, Chemokines, and Growth Factors
Cytokines, chemokines, and growth factors are key mediators of immune system functions capable of signaling through autocrine, paracrine, and endocrine mechanisms. Their pleiotropic immunomodulatory properties allow these biomolecules to react to diverse stimuli and regulate the immune response, either by promoting or inhibiting inflammation. Growing research continues to highlight the role of the immune system in every facet of human health and disease. The MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A and Panel B each offer a unique combination of 48 biomarkers that can be simultaneously analyzed in a small sample volume.
Quality Built into MILLIPLEX® Kits
Kit development and verification entails testing for selectivity and specificity to ensure negligible cross-reactivity in the tested sample types, as well as assay specificity to ensure consistent performance of an assay in single-plex vs. multiplex formats. Buffers and diluents are optimized to enhance antibody specificity, such that only those analytes of interest are detected in samples. The serum matrix is also carefully selected and optimized for use in the standard curve when using serum or plasma samples to most closely mimic sample matrix, thus normalizing assay performance. The streptavidin-phycoerythrin (SAPE) concentration is titrated in-house for optimal signal and is provided ready-to-use with no dilution required. Additionally, all our kits are rigorously tested for shipping stability, and samples are also tested for temperature and freeze/thaw tolerance.
Sample dilution is optimized for each analyte in the kit, ensuring that biologically relevant sample values are detectable, and fall within the dynamic range of the standard curves. Samples tested for this kit include normal and disease serum and plasma samples, as well as peripheral blood mononuclear cell supernatants (PBMCs, unstimulated and stimulated with various agents). Quality Controls (QCs), which are low and high dilutions of recombinant proteins for each analyte, are manufactured such that the two QCs (high and low) have optimal placement on each standard curve. QC range sheets are provided with each kit. Additionally, we always recommend users include experiment-specific samples for use as controls in each assay.
Learn more in our article about quality of MILLIPLEX® kits.
Workflow Improvements
Assays containing 48 individual biomarkers in a single panel provide a powerful tool for research. The ability to select all analytes and narrow them down to a subset of analytes as a project continues allows researchers the flexibility they need to work efficiently. Detecting cytokines at pg/mL levels with standard curve ranges that do not change from lot to lot makes the assay very user-friendly. Consistent standard curves from lot to lot ensure consistent sample results across a project (Figure 1).
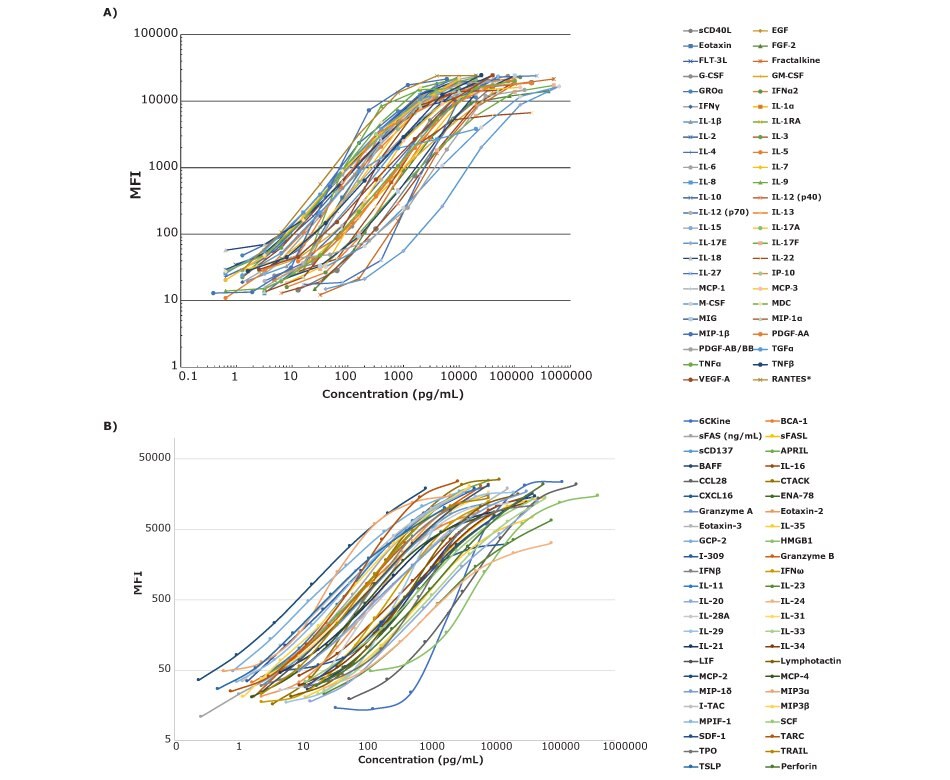
Figure 1.Panel A and B 48-plex standard curves were assayed in serum matrix except *RANTES, which was performed in assay buffer. Each kit was run using the MILLIPLEX® overnight assay protocol.
The MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A and Human Cytokine Panel B have a straightforward protocol with a familiar workflow in which every step is outlined to ensure ease of use.
Another assay improvement to MILLIPLEX® Human Cytokine Panel A is that PDGF-AA and PDGF-AB/BB can now be tested with neat serum and plasma samples, so there is no need for sample dilution as is the case with previous MILLIPLEX® kits or for other Luminex® technology- based kits on the market. RANTES, being highly expressed in serum and plasma samples, still requires a separate sample dilution in serum and plasma of 1:100.
A simple assay improvement of logically arranging the bead regions and analyte names in numeric-alphabetic order, allows researchers to quickly locate specific analytes in the result output files much easier than with previous assays.
Sample Detectability
Human Cytokine Panel A
In addition to improvements in the standard curves relative to the comparative analyte assays for the MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A, there is also improvement in analyte performance in terms of sample detection. One goal in the development of this panel was to closely match sample values when evaluated against comparison MILLIPLEX® kits (Cat. No. HCYTOMAG-60K, HCYP3MAG-63K, or HTH17MAG-14K). Of note, three of the analyte assays have been adjusted to better match the accepted values in the literature: GROα1,2, IL-41, and IL-223,4,5. Figures 2 and 3 show how this panel was used for analyzing disease samples and stimulated PBMCs respectively.
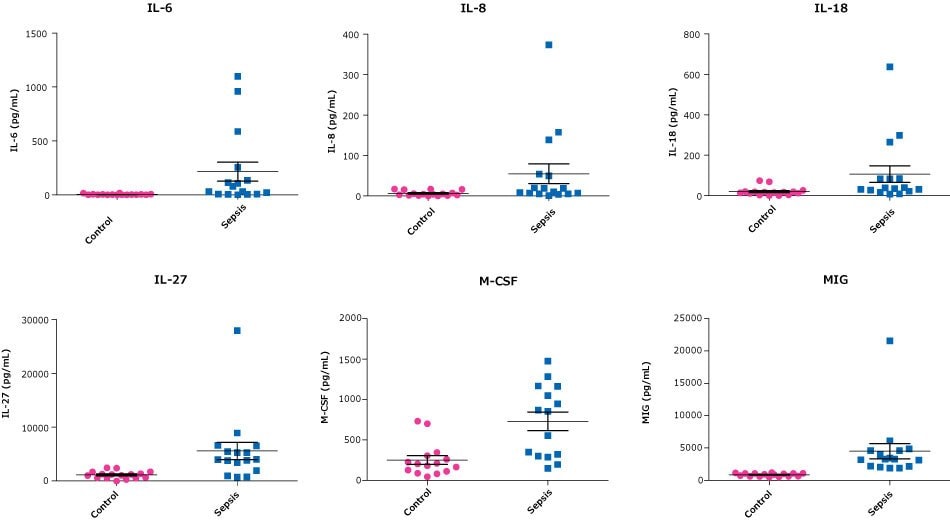
Figure 2.Healthy control serum/plasma samples (obtained from BioIVT) and sepsis patient serum/plasma samples (obtained from BioIVT, Discovery, and BioChemed) were tested neat (25 μL/well) in the MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A. Healthy control serum/plasma samples, N=20. Sepsis serum/plasma samples, N=16.
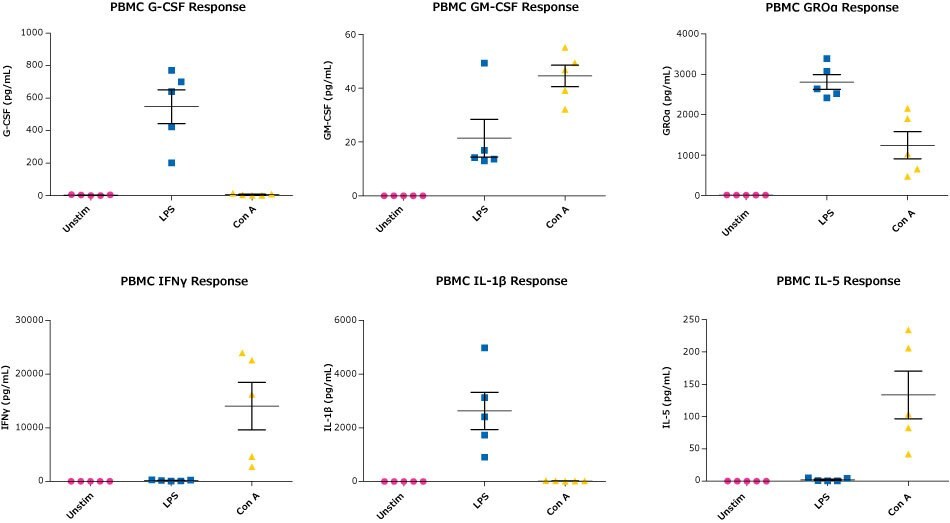
Figure 3.Human PBMC samples (obtained from BioIVT) in RPMI containing 10% FBS and 1% Penicillin/ Streptomycin at 106 cells/mL were incubated with either 1 μg/mL LPS or Con A for 48 hours at 37 °C, after which cell-free supernatants were collected and tested with the MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A.
More details can be found in our MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A application note.
Human Cytokine Panel B
The MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel B 48-plex kit was tested against other Luminex® Brand kits, including Brand 1 which offers a discovery assay and a performance assay that reportedly includes a higher level of assay verification.
In this comparison, the Brand 1 discovery assays are referred to as Brand 1D, and the performance assays are referred to as Brand 1P. MILLIPLEX® Human Cytokine Panel B was also compared to other Luminex® partner kits, here referred to as Brand 2 and Brand 3.
Granzyme B in MILLIPLEX® Human Cytokine Panel B demonstrated 100% sample detectability in both healthy and disease serum and plasma samples (Figure 4A). Conversely, Brand 1P only had 2 detectable disease samples and none of the healthy samples read above background. The discovery assay from the same Brand showed better sample detectability that was comparable in sample range for healthy and disease state samples to MILLIPLEX® Human Panel B. According to a literature search, typical Granzyme B levels should range between 2-50 pg/ mL,6,7 with the MILLIPLEX® panel healthy serum and plasma sample average at 8.9 pg/mL.
For TNF-related apoptosis-inducing ligand (TRAIL), all healthy and disease samples were detectable using MILLIPLEX® Human Cytokine Panel B. Brand 1P had 9/16 (56%) detectable disease samples. As an immune response regulator in sepsis, reduced TRAIL levels in sepsis have been associated with poor outcomes.8 MILLIPLEX® Human Cytokine Panel B followed this trend of reduced TRAIL concentration in disease state samples, including sepsis, which was also demonstrated less prominently in other Brand multiplex panels (Figure 4B). Healthy TRAIL levels have been shown to average around 50-100 pg/mL, which is in line with the MILLIPLEX® Human Cytokine Panel B results.
MIP-3β was detectable in 100% of healthy serum and plasma samples using the MILLIPLEX® Human Cytokine Panel B kit. When compared to other Brand kits, average results were consistent with kits from Brand 2 and Brand 3 and were in line with literature ranges.9 The kits from Brand 1D and 1P demonstrated false positive results in two samples, reading nearly 3-fold higher than the other healthy samples across the 5 multiplex kits (Figure 4C). The kits from Brands 2 and 3 both had detectable levels of MIP-3β in 89% of serum and plasma samples, including disease state samples (data not shown).
CXCL6/GCP-2 demonstrated 100% sample detectability using the MILLIPLEX® Human Cytokine Panel B kit with average values in line with Brand kits 2 and 3. The MILLIPLEX® Panel B was also in line with literature ranges of 40-350 pg/mL.10 As with other analytes, the kit from Brand 1D again showed false positive results with the average healthy sample concentration nearly four times higher than other assays (Figure 4D). Only 55% of healthy samples were detectable using the multiplex assay from Brand 2, which had an overall 58% sample detectability rate when also considering disease state samples (data not shown).
In some instances, sample concentrations in MILLIPLEX® Human Panel B may not match previous kits but are now more in line with literature ranges. This includes analytes like BAFF,11 HMGB1,12 SCF,13 IL-28A,14 and IL-23.15
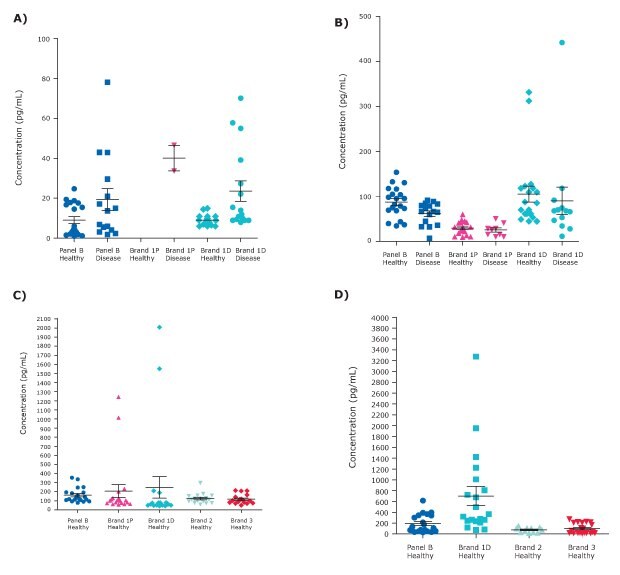
Figure 4.MILLIPLEX® Human Cytokine Panel B sample concentration comparison with other Luminex® Brand multiplex kits. Representative data are shown for (A) Granzyme B, (B) TRAIL, (C) MIP-3β, and (D) CXCL6/GCP-2. Test samples included healthy (n=20) and disease (sepsis and RA; n=16) serum and plasma samples.
More details can be found in our MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel B application note.
For Research Use Only. Not For Use In Diagnostic Procedures.
Recent Publications Using MILLIPLEX® Human Cytokine Panels
- Cytokines and Immune Cell Phenotype in Acute Kidney Injury Associated With Immune Checkpoint Inhibitors. Farooqui N, Zaidi M, Vaughan L, McKee TD, Ahsam E, Pavelko KD, Villasboas JC, Markovic S, Taner T, Leung N, et al. 2022. 8(3):628–641. doi:https://doi.org/10.1016/j.ekir.2022.11.020.
- Clinical and biochemical short-term effects of hyperbaric oxygen therapy on SARS-Cov-2+ hospitalized patients with hypoxemic respiratory failure. Keller GA, Colaianni I, Coria J, Di Girolamo G, Miranda S. 2023. Respiratory Medicine. 209:107155. Doi:https://doi.org/10.1016/j.rmed.2023.107155.
- Plasma Cytokines/Chemokines as Predictive Biomarkers for Lymphedema in Breast Cancer Patients. Vang AR, Shaitelman SF, Rasmussen JC, Chan W, Sevick-Muraca EM, Aldrich MB. 2023. Cancers. 15(3):676. doi:https://doi.org/10.3390/cancers15030676.
- Serum proteomic networks associate with pre-clinical rheumatoid arthritis autoantibodies and longitudinal outcomes. O’Neil LJ, Meng X, Mcfadyen C, Fritzler MJ, El-Gabalawy HS. 2022. Frontiers in Immunology. 13:958145. doi:https://doi.org/10.3389/fimmu.2022.958145.
- The effect of serum IL-2 levels on the prognosis of primary biliary cholangitis-related liver failure and the preliminary exploration of its mechanism. Wang Q, Wang Y, Qiao W, Xu B, Liu Y, Zhang X, Li W, Zhao J, Liu M, Zhang Y, et al. 2022. Frontiers in Immunology. 13:995223. doi:https://doi.org/10.3389/fimmu.2022.995223.
- Transcriptomic Profiling Reveals a Role for TREM-1 Activation in Enterovirus D68 Infection-Induced Proinflammatory Responses. Li J, Yang S, Liu S, Chen Y, Liu H, Su Y, Liu R, Cui Y, Song Y, Teng Y, et al. 2021. Frontiers in Immunology. 12. doi:https://doi.org/10.3389/fimmu.2021.749618.
- Integrative immune transcriptomic classification improves patient selection for precision immunotherapy in advanced gastro-oesophageal adenocarcinoma. Cabeza-Segura M, Gambardella V, Gimeno-Valiente F, Carbonell-Asins JA, Alarcón-Molero L, González-Vilanova A, Zuñiga-Trejos S, Rentero-Garrido P, Villagrasa R, Gil M, et al. 2022. British Journal of Cancer. 127(12):2198–2206. doi:https://doi.org/10.1038/s41416-022-02005-z.
- Epigenetic state determines inflammatory sensing in neuroblastoma.
Wolpaw AJ, Grossmann LD, Dessau JL, Dong MM, Aaron BJ, Brafford PA, Volgina D, Pascual-Pasto G, Rodriguez-Garcia A, Uzun Y, et al. 2022. Proc. Natl. Acad. Sci. U.S.A.. 119(6): https://doi.org/10.1073/pnas.2102358119. - Regulatory T Cells Control Effector T Cell Inflammation in Human Prediabetes. Liu R, Pugh GH, Tevonian E, Thompson K, Lauffenburger DA, Kern PA, Nikolajczyk BS. 2022. 71(2):264-274. https://doi.org/10.2337/db21-0659.
- Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. Ao S, Gao X, Zhan J, Ai L, Li M, Su H, Tang X, Chu C, Han J, Wang F. 2022. Journal of the American Academy of Dermatology. https://doi.org/10.1016/j.jaad.2022.01.039.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?