Chiral LC-MS/MS of D- and L-2-Hydroxyglutaric Acid Biomarkers
Chiral 2-hydroxyglutarates (2-OHG) are important molecular signatures of their specific biochemical pathways and inborn errors of metabolism, for both healthy and diseased biological cells. For example, the TCA cycle of a cancer cell showing the buildup of 2-OHG is depicted in Figure 1. The chiral differentiation and quantification of D-2-OHG and L-2-OHG is key for characterizing neurometabolic disorders like the 2-hydroxyglutaric acidurias that cause neurological impairment early in life.1 In patients with brain tumors, mutations in the enzyme cytosolic isocitrate dehydrogenase 1 (IDH1) are found in approximately 80% of grade II-III gliomas and secondary glioblastomas. The demonstration that cancer-associated IDH1 mutations result in a new ability of the enzyme to catalyze the NADPH-dependent reduction of α-ketoglutarate (2-ketoglutarate) to the oncometabolite D-2-OHG represents a milestone event in cancer biology (see Figure 2).2 Cancer-associated IDH mutations in IDH1 and IDH2 across glioma as well as several hematologic malignancies have become of prognostic interest and for biomarkers and therapeutic opportunities.3
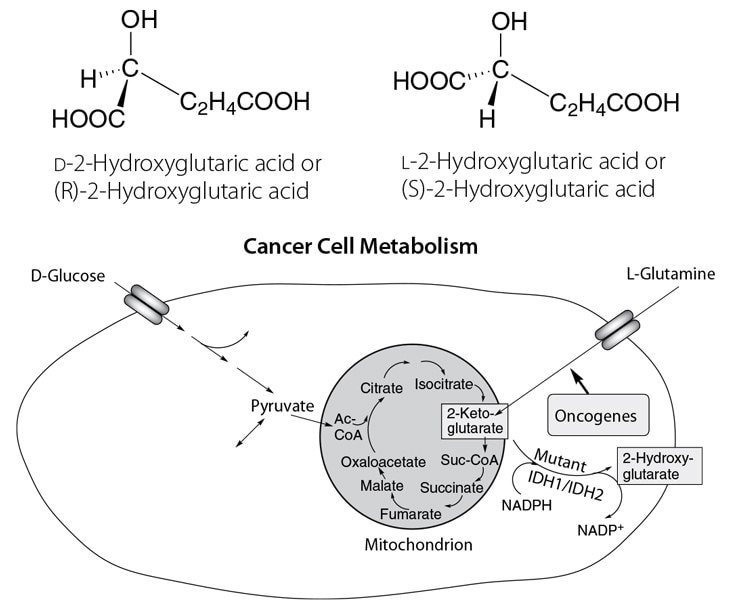
Figure 1. Cancer Cell Metabolism Involving 2-Hydroxyglutaric Acid
The ability to distinguish and quantify the enantiomers of 2-OHG is, therefore, important to be able to track its disposition throughout the metabolic pathway of normal vs. mutated cells (Figure 2). We describe here a rapid and sensitive method to separate and measure D-2-OHG and L-2-OHG using high resolution mass spectrometry (HRMS) detection. An Astec® CHIROBIOTIC® R (ristocetin chiral selector) column was run under polar ionic mobile phase conditions. The separation conditions were based on the work of Rashed, et al., modified in an attempt to improve MS compatibility and separation efficiency.4
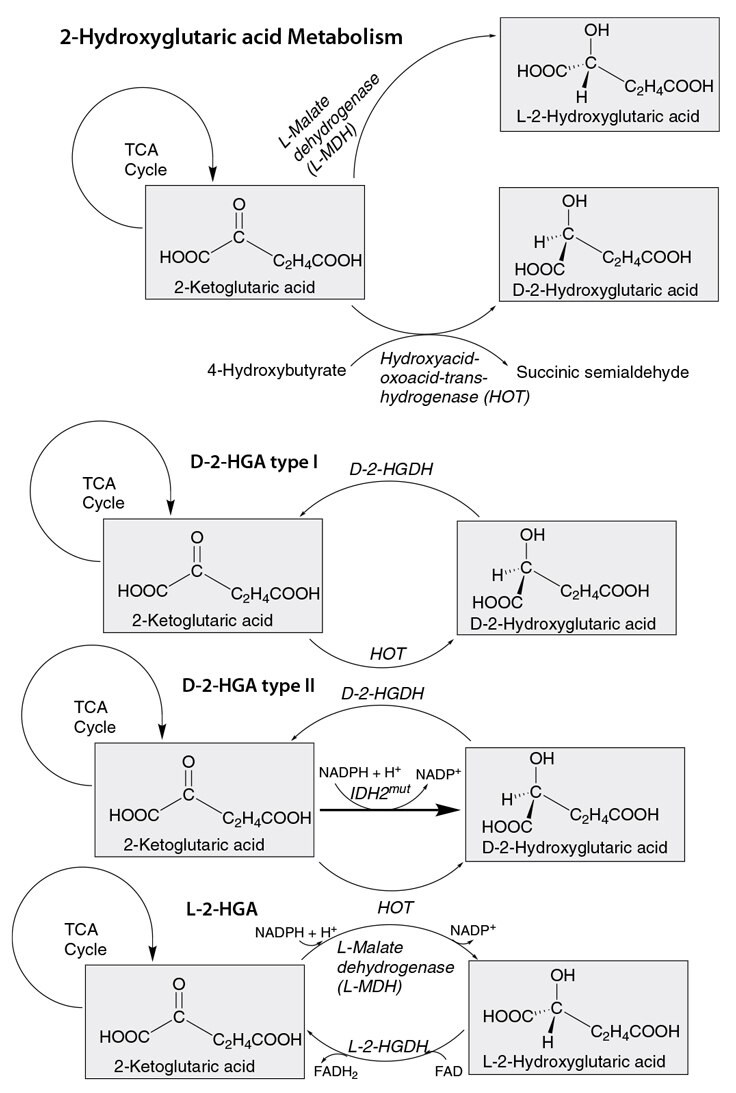
Figure 2. Pathway of 2-Hydroxyglutaric Acid Metabolism
Using HRMS eliminated two fundamental analytical challenges: the lack of a UV-absorbing chromophore in the 2-OHG molecule and the abundance of competing low molecular weight acids in urine. Figure 3 shows the separation of D-2-OHG and L-2-OHG obtained during method development. Besides providing the necessary enantioselectivity, Astec CHIROBIOTIC columns have the advantage of operating in aqueous and polar organic mobile phases that are amenable to polar drugs and metabolites. Retention and selectivity under such conditions promote analyte ionization which enhances sensitivity in ESI detection. In addition to the CHIROBIOTIC columns, Sigma-Aldrich also provides the high purity mobile phase agents, racemic and pure chiral 2-OHG enantiomers and other compounds in the isocitrate dehydrogenase (IDH) pathway.
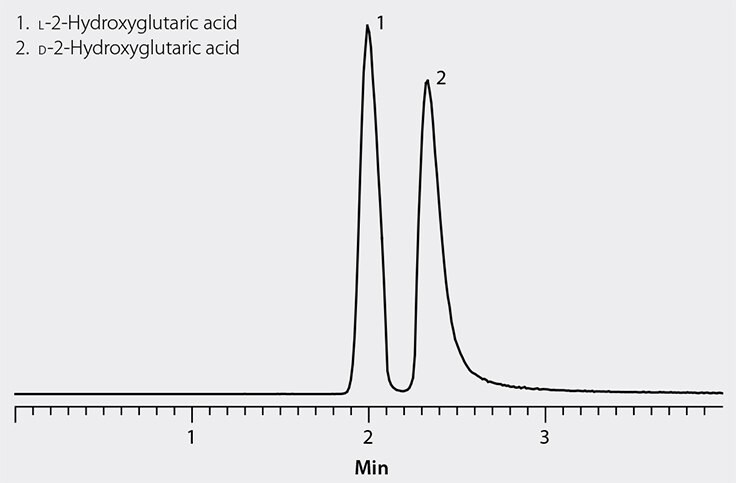
Figure 3. LC-MS Analysis of 2-Hydroxyglutaric Acid Enantiomers on Astec CHIROBIOTIC R
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?