Minipreps using His SpinTrap™ and His SpinTrap™ Kit
His SpinTrap™ and His SpinTrap™ Kit are designed for efficient minipreps (small-scale purification) of histidine-tagged proteins directly from clarified or unclarified cell lysates. The columns may also be used for screening of large numbers of small-scale lysates, as well as optimization of purification conditions. Purification is done in batch mode (by suspension of the medium in the applied sample). The columns are prepacked with Ni Sepharose® High Performance. See Appendix 1 (Characteristics of Ni Sepharose, Ni Sepharose® excel, TALON® Superflow, and uncharged IMAC Sepharose® products) for the main characteristics of His SpinTrap.
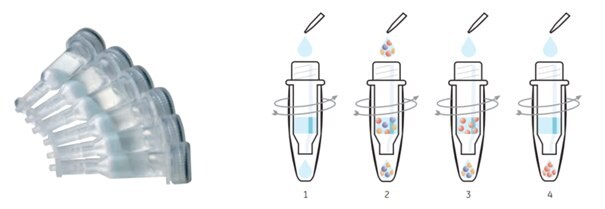
Figure 3.8.His SpinTrap™ is a single-use column for simple, small-scale purification of histidine-tagged proteins and rapid expression screening. Purifying histidine-tagged proteins with His SpinTrap™ is a simple, four-stage procedure that can be performed in 10 min using a microcentrifuge: (1) After placing the column in a 2 mL microcentrifuge tube, equilibrate by adding binding buffer and centrifuge; (2) add sample; (3) wash with binding buffer; (4) elute the target protein with elution buffer.
His SpinTrap™ columns are used together with a standard microcentrifuge. One purification run takes approximately 10 min. For optimal performance, use His SpinTrap™ together with buffers prepared using His Buffer Kit. His Buffer Kit is included in His SpinTrap™ Kit. Purification of unclarified samples on His SpinTrap™ columns minimizes loss of target protein caused by manual operations such as sample precentrifugation, transfer to centrifugation tubes, and collecting supernatant. In addition, loading unclarified sample directly to the His SpinTrap™ columns reduces sample preparation time, which minimizes degradation of sensitive target proteins. Figures 3.8 and 3.9 for schematics showing the procedure.
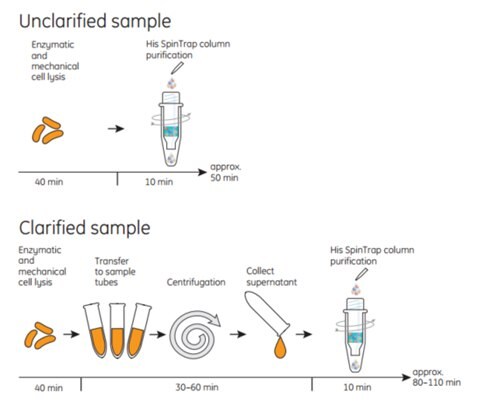
Figure 3.9Total times for preparing and purifying unclarified samples are 30 to 60 min less than for clarified samples because the extra time needed to clarify the cell lysate by centrifugation is eliminated.
Cell Lysis
The procedure below has been used successfully in our own laboratories for sample preparation prior to use of His SpinTrap, but other established procedures may also work. Use standard 2 mL microcentrifuge tubes.
- Dilute the cell paste: Add 1 mL of binding buffer to resuspend cell paste obtained from 20 to 50 mL of cell culture (depending on expression level).
To prevent the binding of host cell proteins with exposed histidines, it is essential to include imidazole at a low concentration in the sample and binding buffer (Chapter 4, Optimizing purification of histidine-tagged proteins).
- a) Enzymatic lysis: Add 0.2 mg/mL lysozyme, 20 µg/mL DNase, 1 mM MgCl2, and 1 mM Pefabloc SC or PMSF. Vortex the tubes gently and incubate at room temperature for 30 min.
Chemical lysis kits can also be used, but make sure that they do not contain any chelating agent.
- b) Mechanical lysis: Disrupt cells by repeated freeze/thaw, homogenization, or sonication.
You can also apply clarified sample to the column by spinning at full speed in a microcentrifuge for 10 min to remove insoluble material. Collect supernatant and purify on His SpinTrap.
Sample and Buffer Preparation
Refer to Purification using Ni Sepharose® High Performance earlier in this chapter, for a general procedure for sample and buffer preparation.
Recommended buffers for native conditions can easily be prepared from His Buffer Kit, which is included in His SpinTrap™ Kit.
Purification
Perform purifications on His SpinTrap™ using a standard microcentrifuge. Place the column in a 2 mL microcentrifuge tube to collect the liquid during centrifugation. Use a new 2 mL tube for every step (steps 1 to 6).
- Invert and shake the column repeatedly to resuspend the medium. Loosen the top cap one-quarter of a turn and break off the bottom closure.
- Place the column in a 2 mL microcentrifuge tube and centrifuge for 30 s at 70 to 100 × g (approx. 1000 rpm in an Eppendorf™ 5415R, 24-position fixed-angle rotor) to remove the storage liquid.
- Remove and discard the top cap. Equilibrate the column by adding 600 µL of binding buffer. Centrifuge for 30 s at 70 to 100 × g.
- Add up to 600 µL (total) of prepared sample. Centrifuge for 30 s at 70 to 100 × g.
You can make several sample applications as long as you do not exceed the binding capacity of the column. See Appendix 1, (Characteristics of Ni Sepharose, Ni Sepharose® excel, TALON® Superflow, and uncharged IMAC Sepharose® products).
- Wash with 600 µL of binding buffer. Centrifuge at 70 to 100 × g for 30 s. Repeat the wash step after sample application if required to obtain sufficient purity.
- Elute the target protein twice with 200 µL of elution buffer. Centrifuge for 30 s at 70 to 100 × g and collect the purified sample. The first 200 µL will contain most of the target protein.
Application Example
Purification of unclarified sample using His SpinTrap
The performance of His SpinTrap™ columns in purifying a histidine-tagged protein from unclarified E. coli lysate was assessed. Histidine-tagged green fluorescent protein, GFP-(His)6, in E. coli BL-21 lysate, was subjected to enzymatic lysis followed by sonication for 10 min, and the unclarified lysate was loaded directly on His SpinTrap. For comparison, half of the sample was also clarified by centrifugation before purification. Samples and binding buffer contained 60 mM imidazole. To ensure complete elution of GFP-(His)6, which has a high affinity for Ni Sepharose® High Performance, the elution buffer contained 800 mM imidazole rather than the more usual 500 mM. Purification time for the unclarified and clarified sample was 10 min. The final purity of eluates from unclarified and clarified samples was similar as confirmed by SDS-PAGE (Figure 3.10).
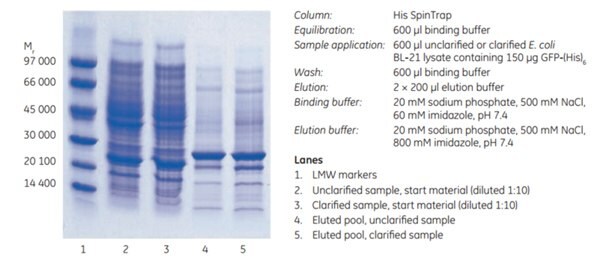
Figure 3.10SDS-PAGE (ExcelGel™ SDS Gradient 8–18) under reducing conditions of unclarified and clarified E. coli lysate containing GFP-(His)6. Similar purity and recovery were observed for both unclarified and clarified sample.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?