Sample Preparation for Size Exclusion Chromatography
Samples for chromatographic purification should be clear and free from particulate matter. Simple steps to clarify a sample before beginning purification will avoid clogging the column, can reduce the need for stringent washing procedures, and can extend the life of the chromatographic medium.
Sample extraction procedures and the selection of buffers, additives, and detergents are determined largely by the source of the material, the stability of the target molecule, the chromatographic techniques that will be employed, and the intended use of the product. These subjects are dealt with in general terms in the Protein Purification Handbook and more specifically according to target molecule in the Recombinant Protein Handbook, and Antibody Purification Handbook, available from Cytiva.
Sample clarification
Centrifugation and filtration are standard laboratory techniques for sample clarification and are used routinely when handling small samples.
It is highly recommended to centrifuge and filter any sample immediately before chromatographic purification.
Centrifugation
Centrifugation removes lipids and particulate matter, such as cell debris. If the sample is still not clear after centrifugation, use filter paper or a 5 μm filter as a first step and one of the filters below as a second step filter.
- For small sample volumes or proteins that adsorb to filters, centrifuge at 10 000 × g for 15 min.
- For cell lysates, centrifuge at 40 000 to 50 000 × g for 30 min.
- Serum samples can be filtered through glass wool after centrifugation to remove any remaining lipids.
Filtration
Filtration removes particulate matter. Membrane filters that give the least amount of nonspecific binding of proteins are composed of cellulose acetate or PVDF.
For sample preparation before chromatography, select a filter pore size in relation to the bead size of the chromatographic medium (Table A3.1).
Check the recovery of the target protein in a test run. Some proteins can adsorb nonspecifically to filter surfaces.
Desalt
Denaturation
Details taken from: Scopes R.K., Protein Purification, Principles and Practice, Springer, (1994), J.C. Janson and L. Rydén, Protein Purification, Principles, High Resolution Methods and Applications, 2nd ed. Wiley Inc, (1998) and other sources.
Precipitation and resolubilization
Specific sample preparation steps might be required if the crude sample is known to contain contaminants such as lipids, lipoproteins, or phenol red, which can build up on a column. Sample preparation can also be required if certain gross impurities, such as bulk protein, need be removed before any chromatographic step.
Fractional precipitation is occasionally used at laboratory scale to remove gross impurities but is generally not required in purification of affinity-tagged proteins. In some cases, precipitation can be useful as a combined protein concentration and purification step.
Precipitation techniques separate fractions by the principle of differential solubility. For example, because protein species differ in their degree of hydrophobicity, increased salt concentrations can enhance hydrophobic interactions between the proteins and cause precipitation. Fractional precipitation can be applied to remove gross impurities in three different ways, as shown in Figure A3.1.
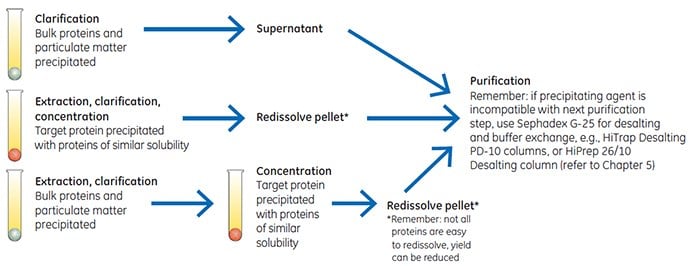
Figure A3.1.Three ways to use precipitaion.
Precipitation techniques can be affected by temperature, pH, and sample concentration.
These parameters must be controlled to ensure reproducible results.
Most precipitation techniques are not suitable for large-scale preparation.
Details taken from: Scopes R.K., Protein Purification, Principles and Practice, Springer, (1994), J.C. Janson and L. Rydén, Protein Purification, Principles, High Resolution Methods and Applications, 2nd ed. Wiley Inc, (1998).
Ammonium sulfate precipitation
Ammonium sulfate precipitation is frequently used for initial sample concentration and clean up. As the concentration of the salt is increased, proteins will begin to “salt out.” Different proteins salt out at different concentrations, a process that can be taken advantage of to remove contaminating proteins from the crude extract. The salt concentration needs to be optimized to remove contaminants and not the desired protein. An additional step with increased salt concentration should then precipitate the target protein. If the target protein cannot be safely precipitated and redissolved, only the first step should be employed. HIC is often an excellent next purification step, as the sample already contains a high salt concentration and can be applied directly to the HIC column with little or no additional preparation. The elevated salt level enhances the interaction between the hydrophobic components of the sample and the chromatography medium.
Solutions needed for precipitation:
- Saturated ammonium sulfate solution (add 100 g ammonium sulfate to 100 mL distilled water, stir to dissolve).
- 1 M Tris-HCl, pH 8.0.
- Buffer for first purification step.
Some proteins can be damaged by ammonium sulfate. Take care when adding crystalline ammonium sulfate: high local concentrations can cause contamination of the precipitate with unwanted proteins.
For routine, reproducible purification, precipitation with ammonium sulfate should be avoided in favor of chromatography.
In general, precipitation is rarely effective for protein concentrations below 1 mg/mL.
- Filter (0.45 μm) or centrifuge the sample (10 000 × g at 4 °C).
- Add 1 part 1 M Tris-HCl, pH 8.0 to 10 parts sample volume to maintain pH.
- Stir gently. Add ammonium sulfate solution, drop by drop. Add up to 50% saturation*. Stir for 1 h.
- Centrifuge for 20 min at 10 000 × g.
- Remove supernatant. Wash the pellet twice by resuspension in an equal volume of ammonium sulfate solution of the same concentration (i.e., a solution that will not redissolve the precipitated protein or cause further precipitation). Centrifuge again.
- Dissolve the pellet in a small volume of the buffer to be used for the next step.
- Ammonium sulfate is removed during clarification/buffer exchange steps with Sephadex G-25 using desalting columns (see Chapter 5).
*The percentage saturation can be adjusted either to precipitate a target molecule or to precipitate contaminants.
The quantity of ammonium sulfate required to reach a given degree of saturation varies according to temperature. Table A3.4 shows the quantities required at 20 °C.
Removal of lipoproteins
Lipoproteins and other lipid material can rapidly clog chromatography columns and it is advisable to remove them before beginning purification. Precipitation agents such as dextran sulfate and polyvinylpyrrolidine, described under Fractional precipitation, are recommended to remove high levels of lipoproteins from samples such as ascites fluid.
Centrifuge samples to avoid the risk of nonspecific binding of the target molecule to a filter.
Samples such as serum can be filtered through glass wool to remove remaining lipids.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?