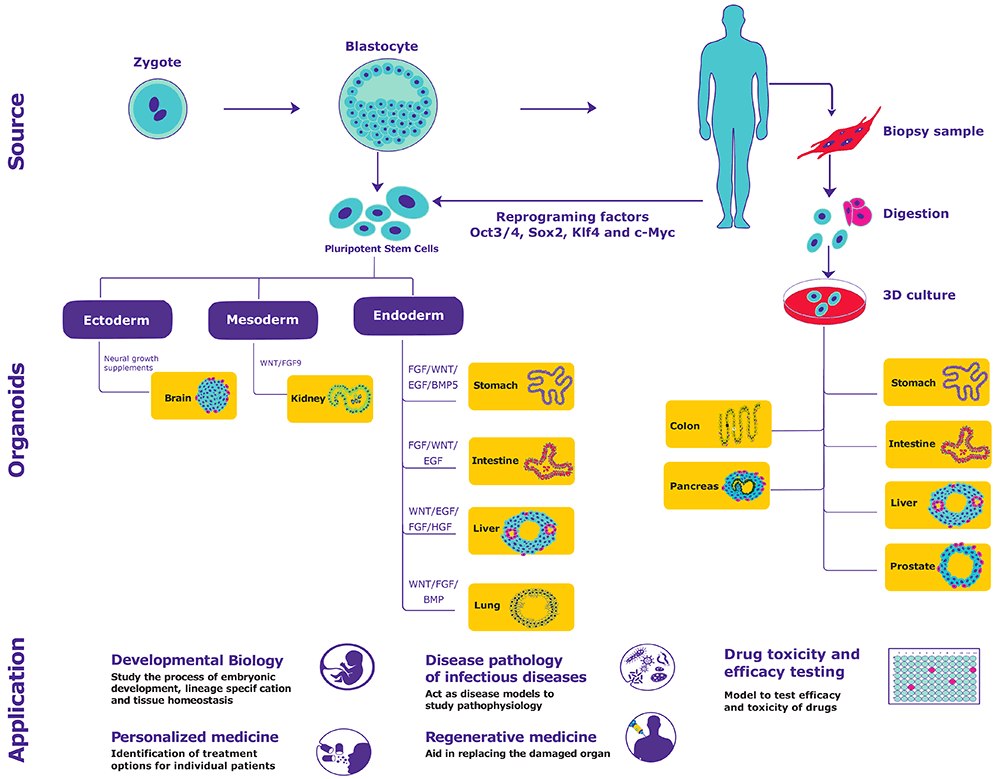
3D类器官培养:用于开发及疾病研究的新体外模型
2D与 3D细胞模型系统的对比
模型系统通过从分子至整个生物体水平概括身体过程与功能来促进生物学研究。人体由以高度专门化方式组织的细胞和非细胞材料组成。用一种体外模型系统很难模拟人类生物学的所有方面。与在2D平面上生长细胞相比,3D细胞培养模型更准确地代表活体生物体中细胞所经历的自然环境。
现有细胞模型系统的局限性
什么是类器官?
类器官是源自原代组织或干细胞的体外衍生3D细胞聚集体,其能够自我更新、自我组织并显示器官功能。3类器官通过提供以下条件解决现有模型系统的局限性:
- 类似于原代组织的组成和结构:类器官含有少量自我更新的干细胞,可以分化成所有主要细胞谱系的细胞,其分化频率与生理条件相似。
- 体内条件的相关模型:类器官与任何模型系统在生物学上更相关,并且能操纵壁龛组分和基因序列。
- 稳定的延伸培养系统:利用干细胞的自我更新、分化能力以及固有的自我组织能力,类器官可冷冻保存用作生物库,亦可无限扩增。
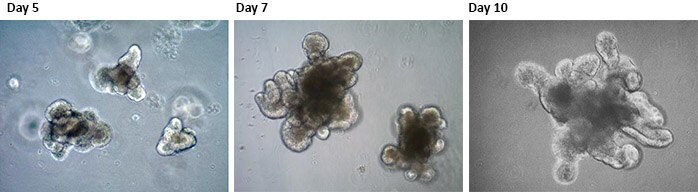
图 1.小鼠小肠上皮类器官按照Clevers等,Clevers et al. Science.2013 概述的实验方案,从成年小鼠肠组织生成3D类器官。类器官细胞在培养的第3-5天开始形成管腔和芽结构,并在第7-10天左右形成复杂的腺窝样结构。这些腺窝样结构区域在功能上类似于成年肠的功能,其中LGR5+ 小肠干细胞的分裂插入位于腺窝基部的潘氏细胞。
类器官和球状体的对比
类器官和类球体都是在3D培养细胞。球状体通常由癌细胞系或肿瘤活检组织形成,为超低附着平板中的自由漂浮细胞聚集体,而类器官则来自嵌入在ECM水凝胶基质(如基质胶)中的组织干细胞。与球装体相比,类器官高度复杂,更接近体内状态。近来,肿瘤类器官已表现出预测患者对癌症药物的反应、帮助制定个性化用药方案方面的作用。
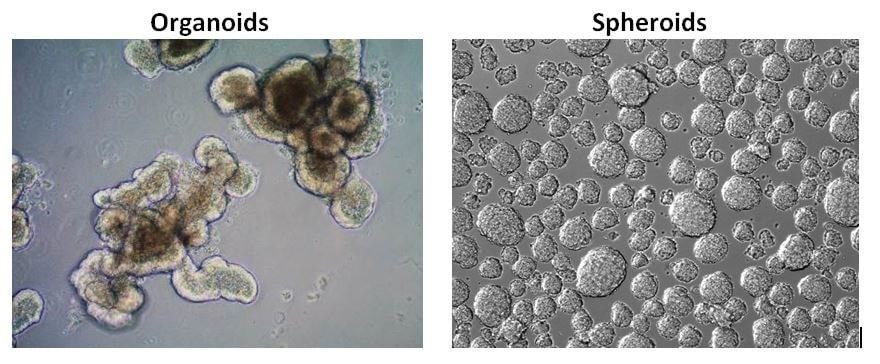
图 2.类器官和球状体的对比。相对于肿瘤球状体,干细胞来源的类器官具有更接近体内的表型,组织复杂程度更高。
类器官是如何产生的?
通过提供适当的物理和生化线索,从原代组织或多能干细胞(诱导的多能干细胞(iPSC)或胚胎干细胞(ESC))产生类器官4。
物理线索:为细胞附着和存活提供支持。实例包括胶原蛋白、纤连蛋白、巢蛋白和层粘连蛋白。
生化线索:调节信号通路,从而影响增殖、分化和自我更新。实例包括EGF、FGF10、HGF、R-spondin、WNT3A、视黄酸、GSK3β抑制剂、TGF-β抑制剂、HDAC抑制剂、ROCK抑制剂、头蛋白、激活素A、p38抑制剂和胃泌素。
通过类器官检验的试剂
类器官的应用
类器官具有生理学相关性,适用于分子和细胞生物学分析,在基础研究和转化应用中具有很大的前景。
发育生物学: 源自ESC和iPSC的类器官保留着其发育阶段的特征,有助于研究胚胎发育过程、谱系规范和组织稳态。发育生物学还阐明了干细胞及其壁龛的发育。
- 通过由调节Wnt、BMP和FGF信号通路诱导的连续分化步骤,研究了脑24、胰腺25和胃7等器官的发育。
传染病的疾病病理学:类器官代表器官的所有组成部分,适用于研究影响特定人类细胞类型的传染病。
- 来自健康儿童的iPSC的肺类器官携带可用于研究流感病毒复制的干扰素调节因子-7基因的无效等位基因26
- 来自人类iPSC的前脑类器官用于研究齐卡病毒对神经祖细胞的感染27
再生医学:移植来自成体干细胞的类器官有助于取代受损的器官或组织。此外,使用CRISPR / Cas9技术进行基因校正的可行性,可用于治疗单基因遗传性疾病。
- 小肠类器官在移植到小鼠模型时保留了小肠的特征,例如绒毛的形成和潘氏细胞的存在28
药物毒性和功效测试:能否测试药物对代表性靶标/器官(肠道、肝脏和肾脏)的功效和毒性,可能会潜在地限制与动物使用相关的伦理问题。
- Hyman肾脏类器官被用来证明顺铂的肾毒性11
个性化药物:来自个体患者的成体干细胞的类器官使得能够对药物反应进行离体测试。
- 结肠类器官用于确定罕见CFTR突变患者的治疗选择29
- 肿瘤类器官可用于评估个体患者水平的药物反应。
相关类器官细胞培养产品
培养器皿
酶
类器官基质
细胞系
培养基及培养试剂
生长因子及细胞介素
小分子
组织学试剂
参考文献
如要继续阅读,请登录或创建帐户。
暂无帐户?