羟醛缩合反应
什么是缩合反应?
羟醛缩合反应是由Charles Wurtz推出的有机反应,他于1872年首次从乙醛中制备了β-羟基醛。1 在羟醛缩合反应中,烯醇离子在酸/碱催化剂存在下与羰基化合物反应,形成β-羟基醛或β-羟基酮,然后脱水得到共轭烯酮。它是一种有用的形成碳-碳键的反应。羟醛缩合反应的基本步骤是:
- 羟醛(醛 + 醇)反应 — 醛(或酮)烯醇化物与另一分子醛(或酮)在NaOH或KOH存在下反应形成β-羟基醛(或酮)。
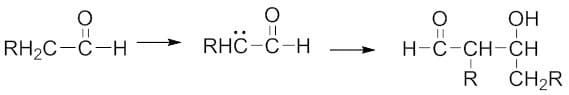
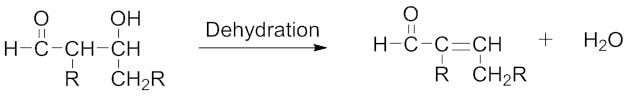
2.脱水/消除反应 — 包括从β-羟基醛(或酮)中除去水分子,形成α,β-不饱和醛或α,β-不饱和酮。
注意事项
请参阅产品化学品安全技术说明书,获取危害和安全操作方法相关信息。
应用
羟醛缩合反应可用于以下合成:
- 酶促合成脂肪酸。2
- 埃博霉素B的高度简洁的全合成。3
- 制备(E)-6-(2,2,3-三甲基-环戊-3-烯基)-己-4-烯-3-酮。4
- 合成聚(戊二醛)的高分子聚合物。5
- (±)-麻黄碱的立体选择性合成。6
- 合成大量大环内酯类和离子载体抗生素(天然产物)。7
- distomadine A和B的全合成,它们是两种结构独特的四环喹诺酮类药物。8
近期研究和趋势
- 水溶性杯[n]芳烃作为反相转移催化剂,用于羟醛类缩合反应以及活化甲基和亚甲基化合物的迈克尔加成反应。9
- 在SBA-15介孔分子载体支架上,含有铯离子的催化剂已用于乙酸甲酯与甲醛的羟醛缩合反应。10
- 已有报道介绍了环己酮与水中的对硝基苯甲醛发生有机催化的不对称羟醛反应。11
- 据报道,一锅铜催化醚化 / 羟醛缩合串联反应产生二苯并氧氮卓内酰胺。12
- 通过烷基化、区域选择性碘化、羟醛缩合、Suzuki偶联和 [1,3]-σ迁移重排来合成一系列二异戊二烯基化和二香叶基化查尔酮类似物。13
- 在甲醇溶剂中,KOH存在下,迈克尔加成反应的多米诺序列以及苊醌与苯乙酮的羟醛缩合反应导致形成不同的2:2加合物。14
- 使用官能化的MCM-41进行了4-异丙基苯甲醛与丙醛的羟醛缩合反应。15
相关产品
Loading
参考文献
1.
2.
Brady RO. 1958. The Enzymatic Synthesis of Fatty Acids by Aldol Condensation. Proceedings of the National Academy of Sciences. 44(10):993-998. https://doi.org/10.1073/pnas.44.10.993
3.
Balog A, Haris C, Savin K, Zhang X, Chou T, Danishefsky S. 1998. Angew. Chem. Int. Ed.. 372675.
4.
Badía C, Castro J, Linares-Palomino P, Salido S, Altarejos J, Nogueras M, Sánchez A. (E)-6-(2,2,3-Trimethyl-cyclopent-3-enyl)-hex-4-en-3-one. Molbank. 2004(1):M388. https://doi.org/10.3390/m388
5.
Tashima T, Imai M, Kuroda Y, Yagi S, Nakagawa T. 1991. Structure of a new oligomer of glutaraldehyde produced by aldol condensation reaction. J. Org. Chem.. 56(2):694-697. https://doi.org/10.1021/jo00002a038
6.
Heathcock CH, Buse CT, Kleschick WA, Pirrung MC, Sohn JE, Lampe J. 1980. Acyclic stereoselection. 7. Stereoselective synthesis of 2-alkyl-3-hydroxy carbonyl compounds by aldol condensation. J. Org. Chem.. 45(6):1066-1081. https://doi.org/10.1021/jo01294a030
7.
Masamune S, Choy W, Kerdesky Francis A. J., Imperiali B. 1981. Stereoselective aldol condensation. Use of chiral boron enolates. J. Am. Chem. Soc.. 103(6):1566-1568. https://doi.org/10.1021/ja00396a050
8.
Jolibois AER, Lewis W, Moody CJ. 2014. Total Synthesis of (±)-Distomadines A and B. Org. Lett.. 16(4):1064-1067. https://doi.org/10.1021/ol403598k
9.
Shimizu S, Shirakawa S, Suzuki T, Sasaki Y. 2001. Water-soluble calixarenes as new inverse phase-transfer catalysts. Their application to aldol-type condensation and Michael addition reactions in water. Tetrahedron. 57(29):6169-6173. https://doi.org/10.1016/s0040-4020(01)00572-5
10.
Yan J, Zhang C, Ning C, Tang Y, Zhang Y, Chen L, Gao S, Wang Z, Zhang W. 2015. Vapor phase condensation of methyl acetate with formaldehyde to preparing methyl acrylate over cesium supported SBA-15 catalyst. Journal of Industrial and Engineering Chemistry. 25344-351. https://doi.org/10.1016/j.jiec.2014.11.014
11.
Mase N, Nakai Y, Ohara N, Yoda H, Takabe K, Tanaka F, Barbas CF. 2006. Organocatalytic Direct Asymmetric Aldol Reactions in Water. J. Am. Chem. Soc.. 128(3):734-735. https://doi.org/10.1021/ja0573312
12.
Lim HS, Choi YL, Heo J. 2013. Synthesis of Dibenzoxepine Lactams via a Cu-Catalyzed One-Pot Etherification/Aldol Condensation Cascade Reaction: Application toward the Total Synthesis of Aristoyagonine. Org. Lett.. 15(18):4718-4721. https://doi.org/10.1021/ol402036t
13.
Wang H, Zhang L, Liu J, Yang Z, Zhao H, Yang Y, Shen D, Lu K, Fan Z, Yao Q, et al. 2015. Synthesis and anti-cancer activity evaluation of novel prenylated and geranylated chalcone natural products and their analogs. European Journal of Medicinal Chemistry. 92439-448. https://doi.org/10.1016/j.ejmech.2015.01.007
14.
Domino reaction sequences leading to the formation of 2:2 adducts between acenaphthenequinone and acetophenone. 2014(6):127. https://doi.org/10.3998/ark.5550190.p008.834
15.
Vrbková E, Vyskocilová E, Cerveny L. 2015. Functionalized MCM-41 as a catalyst for the aldol condensation of 4-isopropylbenzaldehyde and propanal. Reac Kinet Mech Cat. 114(2):675-684. https://doi.org/10.1007/s11144-014-0811-2
登录以继续。
如要继续阅读,请登录或创建帐户。
暂无帐户?