Thermogelation of PLGA-block-PEG-block-PLGA Copolymers
Introduction
Thermogelation is a temperature triggered reversible solution-to-gel phase transition phenomenon. Amphiphilic block copolymers are one class of polymers which display thermogelation behavior. Factors that control thermogelation in amphiphilic block copolymers include molecular weight of block segments, chemical composition of blocks, polymer concentration in solution and end group functionality.1 Therefore, when applied in biomedical applications, the temperature responsive nature of the copolymer can be tuned to induce an in situ gelation at physiological temperature to provide controlled drug release. These materials have been explored for biomedical applications as temperature-responsive biodegradable systems for drug delivery2-5, tissue engineering5 and wound healing6. In controlled release drug delivery the polymer is combined with a therapeutic agent in the solution state and injected into the tissue target. Amphiphilic block copolymers comprised of poly(lactic acid-co-glycolic acid) and poly(ethylene glycol) are thermogelling materials with biodegradable segments. The primary benefit of the thermogel forming biodegradable delivery system is two-fold. First, thermogelation allows for delivery over the course of a period of time after introduction of what is initially a liquid. Second, the material safely biodegrades away once it is no longer necessary to lactic and glycolic acids as well as the water-soluble poly(ethylene glycol) block, which at molecular weights below 20KDa are safely eliminated from the body by the kidneys7. The thermogelation properties of poly(lactic acid-co-glycolic acid)-block-poly(ethylene glycol)-block-poly(lactic acid-co-glycolic acid) (PLGA-block-PEG-block-PLGA) copolymers designed for controlled delivery systems will be described.
Materials Selection
The degradation rate of the copolymers can be tailored by the molecular weight of the degradable block segment and the ratio of lactide to glycolide. Triblock copolymers comprised of PLGA-block-PEG-block-PLGA with relatively low molecular weight were selected for this study. Copolymers with the higher lactide contents (Product No. 764817) decrease the rate of degradation as the additional methyl unit of lactide renders it is more resistant to hydrolysis than copolymers with 1:1 LA:GA molar ratio (Product No. 764787).
Procedure to Incorporate Therapeutic Agent
Polymer Handling/Transfer
These copolymers are extremely viscous (sticky) in their "dry"/neat state (Figure 1), due to their low Tg. The best method to transfer to a new container is to dissolve in the original packaging (e.g. adding 5 mL of saline to ~1 g triblock would yield a 20 % solution) and then transfer the solution of known concentration. The preferred method of transferring an aliquot to another container is to gently warm the gel to ~37 °C for a brief period until it softens to where it can be poured from one container to another. Figure 2 shows the phase physical appearance of the dissolved polymer below, at and above thermogelation transition from solution to gel.

Figure 1.PLGA-PEG-PLGA copolymers in neat state are semi-viscous gels (Product No. 764817 and 764787).

Figure 2.Thermogel appearance at A) ambient or <25 °C; B) upon warming to 25-30 °C; C) >30 °C gelation occurs causing phase separation from solution.
Dissolution
Thermogel forming polymers dissolve best at temperatures below their gel phase transition temperature. For the polymers described herein this means that (counter-intuitively) the copolymer dissolve more rapidly in cold water (or saline solution) (4-10 °C). Do not heat to dissolve. The dissolution takes time; at least overnight, or ideally a few days in advance of when needed. Dissolution can be in either water or saline solution, or mixtures with other ingredients. Stirring and vortex mixing increases the dissolution rate; however, sonication has limited benefit due to increased heating in localized regions of the solution. The polymer solution is stable for 2-3 weeks at 4 °C.
Incorporation of therapeutic agents
The incorporating the desired hydrophobic therapeutic agent can be achieved via water/oil/water (W/O/W) double emulsion methods. The micelle structure of the thermogel polymer enables improved hydrophobic drug solubility. Alternatively, therapeutic agents can be added directly to the aqueous polymer solution and then physically entrapped in the PLGA hydrophobic segments which form physical crosslinks as the temperature of the solution increases and the hydrogen bonding effect of the PEG is decreased limiting its solubility.
Testing
Gelation Testing
Temperature sweep rheometry experiments are utilized to obtain sol-gel curves in order to validate gelation properties of polymer solutions with and without incorporated therapeutic agents. A TA instruments AR550 rheometer was used, equipped with a 60 mm 2° top plate, a peltier temperature controlled bottom plate with a heat-sink water supply maintained at 20 °C by a recirculator. The bottom plate's initial temperature setting was 5 °C with ~ 2 mL of test solution placed in the middle of the bottom plate. The top plate was lowered to a preset gap of 62 μm. Capillary force was used to move the sample into the gap between the plates and was confirmed for proper loading by visual observation. Conditions used for the temperature sweep are shown in Table 1.
Figure 3 illustrates an example sol-gel curve where initially, at low temperature, the liquid solution has no substantial rheological property. However, there is an increase in both G' (red line) and G" (blue line) upon warming to gelation (~23 °C in this graph) along with a decrease in Tan Delta (G’"/G', black line) which indicates the onset of gelation (Figure 3). Upon further heating, the material phase separates out of aqueous solution which returns the rheological curve back to low values for G' and G".
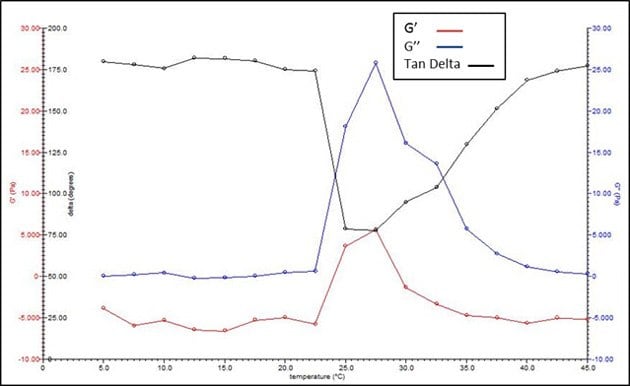
Figure 3.Example thermogelation curve from 10% w/v triblock PLGA-PEG-PLGA (Product No. 764787) in water (without any incorporated agents.
in Vitro Delivery Tests
Amphiphilic block copolymers displaying thermogelation properties have been utilized in several in vitro tests. One method to test performance is to mix a known amount of the gelling solution with a therapeutic agent in a test vial. Warm to 37 °C for a short time (5-10 minutes) to form a gel loaded with the therapeutic agent. Upon gelation, the drug delivery test is started by adding the desired volume of release media and the sample is incubated at 37 °C. Refresh/Test the release media for drug content at desired time-points relevant for your test time period, 1 – 6 weeks.
Conclusion
The use of block copolymers containing poly(ethylene glycol) and poly(lactide-co-glycolide) designed with low overall molecular weight and an appropriate balance between hydrophilic and hydrophobic blocks allows for the formation of biodegradable polymeric materials which can form aqueous solutions that undergo thermogelation at physiological temperatures. This is a useful property for a wide array of applications including drug delivery and tissue engineering.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?